Abstract
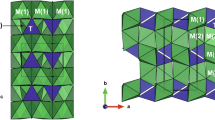
Examination of schorlomite from ijolite at Magnet Cove (USA) and silicocarbonatite at Afrikanda (Russia), using electron-microprobe and hydrogen analyses, X-ray diffraction and Mössbauer spectroscopy, shows the complexity of substitution mechanisms operating in Ti-rich garnets. These substitutions involve incorporation of Na in the eightfold-coordinated X site, Fe2+ and Mg in the octahedrally coordinated Y site, and Fe3+, Al and Fe2+ in the tetrahedrally coordinated Z site. Substitutions Ti4+Fe3+Fe3+−1Si−1 and Ti4+Al3+Fe3+−1Si−1 are of major significance to the crystal chemistry of schorlomite, whereas Fe2+ enters the Z site in relatively minor quantities (<3% Fe∑). There is no evidence (either structural or indirect, such as discrepancies between the measured and calculated Fe2+ contents) for the presence of [6]Ti3+ or [4]Ti4+ in schorlomite. The simplified general formula of schorlomite can be written as Ca3Ti4+2[Si3-x(Fe3+,Al,Fe2+) x O12], keeping in mind that the notion of end-member composition is inapplicable to this mineral. In the published analyses of schorlomite with low to moderate Zr contents, x ranges from 0.6 to 1.0, i.e. Ti4+ in the Y site is <2 and accompanied by appreciable amounts of lower-charged cations (in particular, Fe3+, Fe2+ and Mg). For classification purposes, the mole percentage of schorlomite can be determined as the amount of [6]Ti4+, balanced by substitutions in the Z site, relative to the total occupancy in the Y site: ([6]Ti4+–[6]Fe2+–[6]Mg2+– [8]Na+)/2. In addition to the predominant schorlomite component, the crystals examined in this work contain significant (>15 mol.%) proportions of andradite (Ca3Fe3+2Si3O12), morimotoite (Ca3Fe2+TiSi3O12), and Ca3MgTiSi3O12. The importance of accurate quantitative determination and assignment of Fe, Ti and other cations to the crystallographic sites for petrogenetic studies is discussed.



Similar content being viewed by others
References
Amthauer G, Rossman GR (1984) Mixed valence of iron in minerals with cation clusters. Phys Chem Minerals 11:37–51
Amthauer G, Annersten H, Hafner SS (1977) The Mössbauer spectrum of 57Fe in titanium-bearing andradites. Phys Chem Minerals 1:399-413
Anthony JW, Bideaux RA, Bladh KW, Nichols MC (1995) Handbook of mineralogy, vol II: Silica, silicates. Part 2. Mineral Data Publ, Tucson
Armbruster T, Geiger CA (1993) Andradite crystal chemistry, dynamic X-site disorder and structural strain in silicate garnets. Eur J Mineral 5:59–71
Armbruster T, Birrer J, Libowitzky E, Beran A (1998) Crystal chemistry of Ti-bearing andradites. Eur J Mineral 10:907–921
Basso R, Della Giusta A, Zefiro L (1983) Crystal structure refinement of plazolite: a highly hydrated natural hydrogrossular. Neues Jahrb Mineral Monatsh 1983:251–258
Brod JA, Junqueira-Brod TC, Gaspar JC, Gibson SA, Thompson RN (2003) Ti-rich and Ti-poor garnet from the Tapira carbonatite complex, SE Brazil: Fingerprinting fractional crystallization and liquid immiscibility. In: 8th Inter Kimb Conf Ext Abstr CD, file FLA_0339.pdf
Burns RG (1993) Mineralogical applications of crystal field theory. Cambridge University Press, Cambridge
Chakhmouradian AR, Zaitsev AN (2002) Calcite-amphibole-clinopyroxene rock from the Afrikanda complex, Kola Peninsula, Russia: mineralogy and a possible link to carbonatites. III. Silicate minerals. Can Mineral 40:1347–1374
Chakhmouradian AR, Zaitsev AN (2004) Afrikanda: An association of ultramafic, alkaline and alkali-silica-rich carbonatitic rocks from mantle-derived melts. In: Wall F, Zaitsev AN (eds) Phoscorites and carbonatites from mantle to mine: the key example of the Kola Alkaline Province, Mineralogical Society (UK) Series, 10, pp 247–291
Dawson JB, Smith JV, Steele IM (1995) Petrology and mineral chemistry of plutonic igneous xenoliths from the carbonatite volcano, Oldoinyo Lengai, Tanzania. J Petrol 36:797–826
Deer WA, Howie RA, Zussman J (1982) Rock-forming minerals, vol 1A: Orthosilicates. Longman, New York
Fehr KT, Amthauer G (1996) In: Henmi et al. (1995) (ed) Comment on ‘Morimotoite, Ca3 TiFe2+ Si3O12, a new titanian garnet from Fuka, Okayama Prefecture, Japan. Mineral Mag 60:842–845
Flohr MJK, Ross M (1989) Alkaline igneous rocks of Magnet Cove, Arkansas: Metasomatized ijolite xenoliths from Diamond Jo quarry. Am Mineral 74:113–131
Gaines RV, Skinner HCW, Foord EE, Mason B, Rosenzweig A (1997) Dana’s new mineralogy. Wiley, New York
Grapes RH, Yagi K, Okumura K (1979) Aenigmatite, sodic pyroxene, arfvedsonite and associated minerals in syenites from Morotu, Sakhalin. Contrib Mineral Petrol 69:97–103
Gwalani LG, Rock NMS, Ramasamy R, Griffin BJ, Mulai BP (2000) Complexly zoned Ti-rich melanite-schorlomite garnets from Ambadungar carbonatite-alkalic complex, Deccan Igneous Province, Gujarat State, Western India. J Asian Earth Sci 18:163–176
Hammond AL, Mitchell RH (2002) Accessory mineralogy of orangeite from Swartruggens, South Africa. Mineral Petrol 76:1–19
Henmi C, Kusachi I, Henmi K (1995) Morimotoite, Ca3TiFe2+Si3O12, a new titanian garnet from Fuka, Okayama Prefecture, Japan. Mineral Mag 59:115–120
Holland TJB, Redfern SAT (1997) Unit cell refinement from powder diffraction data: the use of regression diagnostics. Mineral Mag 61:65–77
Howie RA, Woolley AR (1968) The role of titanium and the effect of TiO2 on the cell size, refractive index, and specific gravity in the andradite-melanite-schorlomite series. Mineral Mag 36:775–790
Huggins FE, Virgo D, Huckenholz HG (1977) Titanium-containing silicate garnets. I. The distribution of Al, Fe3+, and Ti4+ between octahedral and tetrahedral sites. Am Mineral 62:475–490
Ibers JA, Hamilton WC, eds (1992) International tables X-ray crystallography, vol IV. Kynoch, Birmingham
Ito J, Frondel C (1967) Synthetic zirconium and titanium garnets. Am Mineral 52:773–781
Kühberger A, Fehr T, Huckenholz HG, Amthauer G (1989) Crystal chemistry of a natural schorlomite and Ti-andradites synthesized at different oxygen fugacities. Phys Chem Minerals 16:734–740
Kukharenko AA, Bagdasarov EA (1962) Paragenesis and crystal-chemical characteristics of titanian garnets from alkali-ultramafic rocks of the Kola Peninsula (in Russian) Uchenye Zapiski LGU. Ser Geolog 13:115
Lager GA, Armbruster T, Faber J (1987) Neutron and X-ray diffraction study of hydrogarnet Ca3Al2(O4H4)3. Am Mineral 72:756–765
Lager GA, Armbruster T, Rotella FJ, Rossman GR (1989) OH substitution in garnets: X-ray and neutron diffraction, infrared, and geometric-modeling studies. Am Mineral 74:840–851
Lehijarvi M (1960) The alkaline district of Iivaara, Kuusamo, Finland. Bull Comm Geol Finl 185:62
Locock A, Luth RW, Cavell RG, Smith DGW, Duke MJM (1995) Spectroscopy of the cation distribution in the schorlomite species of garnet. Am Mineral 80:27–38
Lupini L, Williams CT, Woolley AR (1992) Zr-rich garnet and Zr- and Th-rich perovskite from the Polino carbonatite, Italy. Lithos 56:581–586
Malitesta C, Losito I, Scordari F, Schingaro E (1995) XPS investigation of titanium in melanites from Monte Vulture (Italy). Eur J Mineral 7:847–858
Mill’ BV, Zadneprovskii GM, Bakakin VV (1966) New compounds with structures of the garnet type. Izv Akad Nauk SSSR, Neorg Mater 2:1861–1864
Moore RK, White WB (1971) Intervalence electron charge transfer effects in the spectra of the melanite garnets. Am Mineral 56:826–840
Müntener O, Hermann J (1994) Titanian andradite in a metapyroxenite layer from the Malenco ultramafics (Italy): implications for Ti-mobility and low oxygen fugacity. Contrib Mineral Petrol 116:156–168
Peterson RC, Locock AJ, Luth RW (1995) Positional disorder of oxygen in garnet: the crystal-structure refinement of schorlomite. Can Mineral 33:627–631
Povarennykh AS, Shabalin BG (1983) Structural role of titanium and iron in synthetic zirconium- and titanium-bearing garnets (in Russian). Geol Zhurnal 43:45–50
Rass IT (1997) Morimotoite, a new titanian garnet?—discussion. Mineral Mag 61:728–730
Rass IT, Dubrovinskii LS (1997) The thermodynamic parameters and stability region of schorlomite. Trans (Doklady) Russ Acad Sci Earth Sci Sect 355:730–732
Rickwood PC (1968) On recasting analyses of garnet into end-member molecules. Contrib Mineral Petrol 18:175–198
Roberts WL, Rapp GR, Weber J (1974) Encyclopedia of Minerals. Van Nostrand Reinhold, New York
Russell JK, Dipple GM, Lang JR, Lueck B (1999) Major-element discrimination of titanian andradite from magmatic and hydrothermal environments: an example from the Canadian Cordillera. Eur J Mineral 11:919–935
Schingaro E, Scordari F, Capitanio F, Parodi G, Smith DC, Mottana A (2001) Crystal chemistry of kimzeyite from Anguillara, Mts. Sabatini, Italy. Eur J Mineral 13:749–759
Schwartz KB, Nolet DA, Burns RG (1980) Mössbauer spectroscopy and crystal chemistry of natural Fe–Ti garnets. Am Mineral 65:142–153
Scordari F, Schingaro E, Pedrazzi G (1999) Crystal chemistry of melanites from Mt. Vulture (Southern Italy). Eur J Mineral 11:855–869
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystal A32:751–767
Shepard CU (1846) On three new mineral species from Arkansas, and the discovery of the diamond in North Carolina. Am J Sci 2:249–254
Ulrych J, Povondra P, Pivec E, Rutšek J, Sitek J (1994) Compositional evolution of metasomatic garnet in melilitic rocks of the Osečná complex, Bohemia. Can Mineral 32:637–647
Waychunas GA (1987) Synchrotron radiation XANES spectroscopy of Ti in minerals: effects of Ti bonding distances, Ti valence, and site geometry on absorption edge structure. Am Mineral 72:89–101
Wu G, Mu B (1986) The crystal chemistry and Mössbauer study of schorlomite. Phys Chem Minerals 13:198–205
Yakovenchuk VN, Ivanyuk GYu, Pakhomovskiy YaA, Men’shikov YuP (1999) Minerals of the Khibina Massif (in Russian). Zemlya, Moscow
Yakovlevskaya TA (1972) The garnet group (in Russian). In: Chukhrov FV (ed) Minerals. vol III, Issue 1. Nauka, Moscow, pp 17–85
Zedlitz O (1933) Über titanreichen Kalkeisengranat. Zentrabl Mineral 1933A:225–239
Acknowledgements
This work was supported in part by the Natural Sciences and Engineering Research Council of Canada (ARC). Mössbauer experiments were performed using the equipment purchased with funds awarded to F. Seifert by Fonds der Chemischen Industrie (Germany) under the “Materials Research” program. Mike Howard is cordially thanked for providing us with a collection of Magnet Cove garnets (including sample MC-04) and details on their geological provenance. We are very grateful to Mark Cooper for his help with the collection, processing and interpretation of the single-crystal data, and for his comments on the earlier draft of this paper. This study would not have been possible without the kind permission of Frank C. Hawthorne to use the single-crystal X-ray facility at the University of Manitoba, equipped through Major Equipment and Equipment grants from the Natural Sciences and Engineering Research Council of Canada to FCH. We are also grateful to Ron Chapman, Keith Pringnitz and Allan MacKenzie for their help with the analytical work, and to Anne Hammond for the masterfully executed thin sections. This manuscript has benefited from many constructive comments made by two anonymous reviewers, Giovanni Ferraris and editor James Tyburczy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chakhmouradian, A.R., McCammon, C.A. Schorlomite: a discussion of the crystal chemistry, formula, and inter-species boundaries. Phys Chem Minerals 32, 277–289 (2005). https://doi.org/10.1007/s00269-005-0466-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-005-0466-7