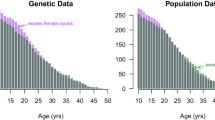
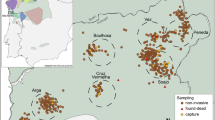
Abstract
Offspring sex ratios can vary widely across species, and the reasons for such variation have long intrigued ecologists. For group-living animals, predicting offspring sex ratios as a function of group and environmental characteristics can be challenging. Additionally, mortality of group members can upend traditional theory used to explain offspring sex ratios observed in populations. Gray wolves (Canis lupus) in Idaho, USA, are an excellent study species for asking questions about offspring sex ratios given their group-living behavior and persistent exposure to human-caused mortality. I hypothesized that offspring sex ratios would be influenced by the characteristics of individuals, groups, and populations. I generated genotypes for 419 adult and 400 pup wolves during 2008–2018. There was a significant male-bias in litters of wolf pups with nearly 12% more male pups born than females. The individual, group, and population variables I considered did not have significant associations with offspring sex ratios. Local resource competition helped explain offspring sex ratios in wolves in my study system, but not local resource enhancement theory. Although female helpers have been shown to help slightly more than males, offspring sex ratios did not favor the helping sex suggesting that the overall benefit of female helpers may have been negligible in wolf groups during my study. Three wolf groups consistently overproduced males, the dispersing sex, suggesting that habitat quality was poor in their territories. The male-biased offspring sex ratios observed throughout this population reflect sex-biased dispersal in wolves in Idaho. Such a pattern suggests breeding females may be reducing local resource competition (e.g., mates and successful reproduction) by producing more males than females.
Significance statement
Natural selection can favor biased offspring sex ratios in some species. This may be particularly true for animals that live and breed in groups such as gray wolves. Using genetic sampling, I show that offspring sex ratios in wolves are male-biased and reflect sex-biased dispersal in wolves. Breeding females may be reducing future local resource competition for mates by producing significantly more offspring of the dispersing sex (males).


Similar content being viewed by others
Data availability
Ausband DE (2022b) Wolf pup sex ratios and covariates, Idaho, USA, 2008–2018 [Data set]. Zenodo, https://doi.org/10.5281/zenodo.7035926
References
Almberg ES, Cross PC, Smith DW (2010) Persistence of canine distemper virus in the Greater Yellowstone Ecosystem’s carnivore community. Ecol Appl 20:2058–2074
Ausband DE (2016) Gray wolf harvest in Idaho. Wildlife Soc B 40:500–505
Ausband DE (2021) Mate selection data for gray wolves. Zenodo. https://doi.org/10.5281/zenodo.5764792
Ausband DE (2022a) Inherit the kingdom or storm the castle? Breeding strategies in gray wolves. Ethology 128:152–158
Ausband DE (2022b) Wolf pup sex ratios and covariates, Idaho, USA 2008–2018. Zenodo. https://doi.org/10.5281/zenodo.7035926
Ausband DE, Mitchell MS, Bassing SB, Morehouse A, Smith DW, Stahler DR, Struthers J (2016) Individual, group, and environmental influences on helping behavior in a social carnivore. Ethology 122:963–972
Ausband DE, Mitchell MS, Doherty K, Zager P, Mack CM, Holyan J (2010) Surveying predicted rendezvous sites to monitor gray wolf populations. J Wildlife Manage 74:1043–1049
Ausband DE, Mitchell MS, Waits L (2017) Effects of breeder turnover and harvest on group composition and recruitment in a social carnivore. J Anim Ecol 86:1094–1011
Breen M, Jouquand S, Renier C et al (2001) Chromosome-specific single-locus FISH probes allow anchorage of an 1800-marker integrated radiation-hybrid/linkage map of the domestic dog genome to all chromosomes. Genome Res 11:1784–1795
Creel S, Marusha Creel N, Monfort SL (1998) Birth order, estrogens and sex-ratio adaptation in African wild dogs (Lycaon pictus). Anim Reprod Sci 53:315–320
Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford
Guyon R, Lorentzen TD, Hitte C et al (2003) A 1-Mb resolution radiation hybrid map of the canine genome. Proc Natl Acad Sci USA 100:5296–5301
Hamilton WD (1967) Extraordinary sex ratios. Science 156:477–488
Harrington FH, Mech LD (1982) An analysis of howling response parameters useful for wolf pack censusing. J Wildlife Manage 46:686–693
Holand Ø, Mysterud A, Røed KH, Coulson T, Gjøstein H, Weladji RB, Nieminen M (2006) Adaptive adjustment of offspring sex ratio and maternal reproductive effort in an iteroparous mammal. Proc R Soc Lond B 273:293–299
Holmes NG, Strange NJ, Binns MM, Mellersh CS, Sampson J (1994) Three polymorphic canine microsatellites. Anim Genet 25:200
Iossa G, Soulsbury CD, Baker PJ, Edwards KJ, Harris S (2009) Behavioral changes associated with a population density decline in the facultatively social red fox. Behav Ecol 20:385–395
Jacobs C, Ausband DE (2019) Wolves in space: locations of individuals and their effect on pup survival in groups of a cooperatively breeding canid. Anim Behav 155:189–197
Jimenez MD, Bangs EE, Boyd DK, Smith DW, Becker SA, Ausband DE, Woodruff SP, Bradley EH, Holyan J, Laudon K (2017) Wolf dispersal in the northern Rocky Mountains, Western United States: 1993–2008. J Wildlife Manage 81:581–592
Jones O, Wang J (2009) COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour 10:551–555
Julliard R (2000) Sex-specific dispersal in spatially varying environments leads to habitat-dependent evolutionary stable offspring sex ratios. Behav Ecol 11:421–428
Kreeger TJ (2003) The internal wolf: Physiology, pathology, and pharmacology. In: Mech LD, Boitani L (eds) Wolves: ecology, conservation, and management. The University of Chicago Press, IL, USA, pp 192–217
Li J, Wang Y, Lv L, Wang P, Zhang Z (2016) No facultative manipulation of offspring sex ratio in relation to parental genetic characteristics in a bird with sex-specific heterozygosity-fitness correlation. Behav Ecol Sociobiol 70:963–973
McNutt JW, Silk JB (2008) Pup production, sex ratios, and survivorship in African wild dogs, Lycaon pictus. Behav Ecol Sociobiol 62:1061–1067
Mech LD (1975) Disproportionate sex ratios of wolf pups. J Wildlife Manage 39:737–740
Mech LD, Goyal SM, Paul WJ, Newton WE (2008) Demographic effects of canine parvovirus on a free-ranging wolf population over 30 years. J Wildlife Dis 44:824–836
Miller P (2017) Population viability analysis for the Mexican wolf (Canis lupus baileyi) integrating wild and captive populations in a metapopulation risk assessment model for recovery planning. In: Biological report for the Mexican wolf (Canis lupus baileyi) November 2017. U.S. Fish and Wildlife Service, Southwest Region (Region 2), Albuquerque, NM, USA, 72 p
Mills LS (2013) Conservation of wildlife populations: demography, genetics, and management, 2nd edn. Wiley-Blackwell, Oxford
Morales-Gonzalez A, Fernandez-Gil A, Quevedo M, Revilla E (2021) Patterns and determinants of dispersal in grey wolves (Canis lupus). Biol Rev 97:466–480
Ostrander EA, Wayne RK, Freedman AH, Davis BW (2017) Demographic history, selection and functional diversity of the canine genome. Nat Rev Genet 18:705–720
R Development Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org. Accessed 5 July 2022
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol 6:288–295
Salim DC, Akimoto AA, Carvalho CB, Oliveir SF, Grisolia CK, Moreira JR, Klautau-Guimarães MN (2007) Genetic variability in maned wolf based on heterologous short-tandem repeat markers from domestic dog. Genet Mol Res 6:348–435
Sidorovich VE, Stolyarov VP, Vorobei NN, Ivanova NV, Jedrzejewska B (2007) Litter size, sex ratio, and age structure of gray wolves, Canis lupus, in relation to population fluctuations in northern Belarus. Can J Zool 85:295–300
Silk JB, Brown GR (2008) Local resource competition and local resource enhancement shape primate birth sex ratios. Proc R Soc Lond B 275:1760–1765
Stansbury CS, Ausband DE, Zager P, Mack CM, Miller CR, Pennell MW, Waits LP (2014) A long-term population monitoring approach for a wide-ranging carnivore: noninvasive genetic sampling of gray wolf rendezvous sites in Idaho, USA. J Wildlife Manage 78:1040–1049
Stenglein JL, De Barba M, Ausband DE, Waits LP (2010a) Impacts of sampling location within a faeces on DNA quality in two carnivore species. Mol Ecol Resour 10:109–114
Stenglein JL, Waits LP, Ausband DE, Zager P, Mack CM (2010b) Efficient noninvasive genetic sampling for monitoring reintroduced wolves. J Wildlife Manage 74:1050–1058
Stenglein JL, Waits LP, Ausband DE, Zager P, Mack C (2011) Estimating gray wolf pack size and family relationships using noninvasive genetic sampling at rendezvous sites. J Mammal 92:784–795
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
vonHoldt BM, Stahler DR, Smith DW, Earl DA, Pollinger JP, Wayne RK (2008) The genealogy and genetic viability of reintroduced Yellowstone grey wolves. Mol Ecol 17:252–274
Wang J (2011) COANCESTRY: a program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol Ecol Resour 11:141–145
Western Regional Climate Center (2022) Historical climate information. http://www.wrcc.dri.edu. Accessed 11 Mar 2022
Acknowledgements
I thank M.A. Hurley, J.S. Husseman, S.B. Roberts, C.R. Stansbury, J.L. Stenglein, J.L. Struthers, J. Adams, and L. Waits for their assistance and S.B. Bassing for the manuscript review. I also thank field technicians for the perseverance in the field while collecting these data. Lastly, I thank anonymous reviewers for their thoughtful and thorough reviews. During the data collection, I received financial support from the Regina Bauer Frankenberg Foundation for Animal Welfare, Bernice Barbour Foundation, Coypu Foundation, Eppley Foundation for Research, Idaho Department of Fish and Game, Kampe Foundation, Leonard X. Bosack and Bette M. Kruger Charitable Foundation, Nancy Carroll Draper Foundation, Nez Perce Tribe, Oregon Zoo Future for Wildlife grants, Shikar Safari Club International Foundation, Steven Leuthold Family Foundation, The Mountaineers Foundation, U.S. Fish and Wildlife Service, Wilburforce Foundation, Wolf Recovery Foundation, University of Idaho College of Natural Resources, and University of Idaho Environmental Science Program. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This paper was subject to USGS Fundamental Science Practices (https://pubs.usgs.gov/circ/1367).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Field sampling was conducted under the University of Montana IACUC (Animal Use Protocol 008-09MMMCWRU). All applicable international, national, and/or institutional guidelines for the use of animals were followed.
Conflict of interest
The author declares no competing interests.
Additional information
Communicated by C. Soulsbury
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ausband, D.E. Offspring sex ratios are male-biased reflecting sex-biased dispersal in Idaho, USA, wolves. Behav Ecol Sociobiol 76, 134 (2022). https://doi.org/10.1007/s00265-022-03243-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03243-0