Abstract
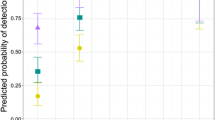
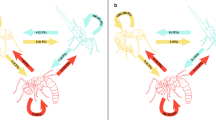
Polymorphism frequently correlates with specialized labor in social insects, but extreme morphologies may compromise behavioral flexibility and thus limit caste evolution. The ant genus Pheidole has dimorphic worker subcastes in which major workers appear limited due to their morphology to performing defensive or trophic functions, thus providing an ideal model to investigate specialization and plasticity. We examined worker morphology, brood-care flexibility, and subcaste ratio in 17 species of tropical twig-nesting Pheidole by quantifying nursing by major workers in natural colonies and in subcolonies lacking minors, in which we also measured brood survival and growth. Across species, majors performed significantly less brood care than minors in intact colonies, but increased rates of brood care 20-fold in subcolonies lacking minors. Brood nursed by majors had lower survival than brood tended by minors, although rates of brood growth did not vary between subcastes. Significant interspecific variation in rates of brood care by major workers did not lead to significant differences in brood growth or survival. Additionally, we did not find a significant association between the degree of major worker morphometric specialization and rates of nursing, growth, or survival of brood among species. Therefore, major workers showed reduced efficacy of brood care, but the degree of morphological specialization among species did not directly compromise task plasticity. The compact nests and all-or-nothing consequences of predation or disturbance on colony fitness may have influenced the evolution of major worker brood-care competency in twig-nesting Pheidole.





Similar content being viewed by others
References
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300
Bernays EA (1998) The value of being a resource specialist: behavioral support for a neural hypothesis. Am Nat 151:451–464
Bernays EA, Wcislo WT (1994) Sensory capabilities, information processing, and resource specialization. Q Rev Biol 69:187–204
Bolton B, Alpert G, Ward PS, Naskrecki P (2006) Bolton’s catalogue of ants of the world 1758–2005. Harvard University Press, Cambridge, MA
Braendle C, Hockley N, Brevig T, Shingleton AW, Keller L (2003) Size-correlated division of labour and spatial distribution of workers in the driver ant, Dorylus molestus. Naturwissenschaften 90:277–281
Brandão CRF (1978) Division of labor within the worker caste of Formica peripilosa Wheeler (Hymenoptera: Formicidae). Psyche 85:229–238
Brown MB, Forsythe AB (1974) Robust tests for the equality of variances. J Am Stat Assoc 69:364–367
Brown JJ, Traniello JFA (1998) Regulation of brood-care behavior in the dimorphic castes of the ant Pheidole morrisi (Hymenoptera: Formicidae): effects of caste ratio, colony size, and colony needs. J Insect Behav 11:209–219
Busher CE, Calabi P, Traniello JFA (1985) Polymorphism and division of labor in the Neotropical ant Camponotus sericeiventris Guerin (Hymenoptera: Formicidae). Ann Entomol Soc Am 78:221–228
Byrne MM (1994) Ecology of twig-dwelling ants in a wet lowland tropical forest. Biotropica 26:61–72
Caillaud MC, Via S (2000) Specialized feeding behavior influences both ecological specialization and assortative mating in sympatric host races of pea aphids. Am Nat 156:606–621
Calabi P, Traniello JFA (1989) Social organization in the ant Pheidole dentata. Behav Ecol Sociobiol 24:69–78
Carlin NF, Johnston AB (1984) Learned enemy specification in the defense recruitment system of an ant. Naturwissenschaften 71:156–157
Cassill DL, Tschinkel WR (1995) Allocation of liquid food to larvae via trophallaxis in colonies of the fire ant, Solenopsis invicta. Anim Behav 50:801–813
Cassill DL, Butler J, Vinson SB, Wheeler DE (2005) Cooperation during prey digestion between workers and larvae in the ant, Pheidole spadonia. Insectes Soc 52:339–343
Chang VCS (1985) Colony revival, and notes on rearing and life history of the big-headed ant. Proc Hawaii Entomol Soc 25:53–58
Conover WJ, Johnson ME, Johnson MM (1981) A comparative study of tests for homogeneity of variances, with applications to the outer continental shelf bidding data. Technometrics 23:351–361
Davidowitz G, D’Amico LJ, Nijhout HF (2003) Critical weight in the development of insect body size. Evol Dev 5:188–197
Davidson DW (1977) Species diversity and community organization in desert seed-eating ants. Ecology 58:711–724
Davidson DW (1978) Size variability in the worker caste of a social insect (Veromessor pergandei Mayr) as a function of the competitive environment. Am Nat 112:523–532
Detrain C, Pasteels JM (1992) Caste polyethism and collective defense in the ant, Pheidole pallidula: the outcome of quantitative differences in recruitment. Behav Ecol Sociobiol 29:405–412
Dornhaus A (2008) Specialization does not predict individual efficiency in an ant. PLoS Biol 6:e285
Dyer LA, Singer MS, Lill JT, Stireman JO, Gentry GL, Marquis RJ, Ricklefs RE, Greeney HF, Wagner DL, Morais HC (2007) Host specificity of Lepidoptera in tropical and temperate forests. Nature 448:696
Fjerdingstad EJ, Crozier RH (2006) The evolution of worker caste diversity in social insects. Am Nat 167:390–400
Fry JD (1996) The evolution of host specialization: are trade-offs overrated? Am Nat 148:S84–S107
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Gilbert F, Jervis M (1998) Functional, evolutionary and ecological aspects of feeding-related mouthpart specializations in parasitoid flies. Biol J Linn Soc 63:495–535
Gotelli NJ, Ellison AM (2004) A primer of ecological statistics. Sinauer Associates, Sunderland, MA
Gotwald WH Jr (1978) Trophic ecology and adaptation in tropical old world ants of the subfamily Dorylinae (Hymenoptera: Formicidae). Biotropica 10:161–169
Gronenberg W, Paul J, Just S, Hölldobler B (1997) Mandible muscle fibers in ants: Fast or powerful? Cell Tissue Res 289:347–361
Hansen SR (1978) Resource utilization and coexistence of three species of Pogonomyrmex ants in an upper sonoran grassland community. Oecologia 35:109–117
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge, MA
Jaenike J (1990) Host specialization in phytophagous insects. Annu Rev Ecol Syst 21:243–273
Johnson RA (2001) Biogeography and community structure of North American seed-harvester ants. Annu Rev Entomol 46:1–29
Johnson BR (2003) Organization of work in the honeybee: a compromise between division of labour and behavioural flexibility. Proc R Soc Lond B Biol Sci 270:147–152
Johnson BR (2005) Limited flexibility in the temporal caste system of the honey bee. Behav Ecol Sociobiol 58:219–226
Johnston AB, Wilson EO (1985) Correlates of variation in the major/minor ratio of the ant, Pheidole dentata (Hymenoptera: Formicidae). Ann Entomol Soc Am 78:8–11
Julian GE, Gronenberg W (2002) Reduction of brain volume correlates with behavioral changes in queen ants. Brain Behav Evol 60:152–164
Karsai I, Wenzel JW (1998) Productivity, individual-level and colony-level flexibility, and organization of work as consequences of colony size. Proc Acad Nat Sci Philadelphia 95:8665–8669
Kaspari M (1996a) Litter ant patchiness at the 1-m2 scale: disturbance dynamics in three Neotropical forests. Oecologia 107:265–273
Kaspari M (1996b) Worker size and seed size selection by harvester ants in a Neotropical forest. Oecologia 105:397–404
Kaspari M, Byrne MM (1995) Caste allocation in litter Pheidole: lessons from plant defense theory. Behav Ecol Sociobiol 37:255–263
Kay A, Rissing SW (2005) Division of foraging labor in ants can mediate demands for food and safety. Behav Ecol Sociobiol 58:165–174
Larsson M (2005) Higher pollinator effectiveness by specialist than generalist flower-visitors of unspecialized Knautia arvensis (Dipsacaceae). Oecologia 146:394–403
MacArthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101:377–385
McGlynn TP, Owen JP (2002) Food supplementation alters caste allocation in a natural population of Pheidole flavens, a dimorphic leaf-litter dwelling ant. Insectes Soc 49:8–14
Mehlhop P, Scott NJ (1983) Temporal patterns of seed use and availability in a guild of desert ants. Ecol Entomol 8:69–85
Mertl AL, Ryder Wilkie KT, Traniello JFA (2009) Impact of flooding on the species richness, density and composition of Amazonian litter-nesting ants. Biotropica. doi:10.1111/j.1744-7429.2009.00520.x
Mery F, Kawecki TJ (2003) A fitness cost of learning ability in Drosophila melanogaster. Proc R Soc Lond B Biol Sci 270:2465–2469
Mery F, Kawecki TJ (2004) An operating cost of learning in Drosophila melanogaster. Anim Behav 68:589–598
Moreau CS (2008) Unraveling the evolutionary history of the hyperdiverse ant genus, Pheidole (Hymenoptera: Formicidae). Mol Phylogenet Evol 48:224–239
Muscedere ML, Willey TA, Traniello JFA (2009) Age and task efficiency in the ant Pheidole dentata; young minor workers are not specialist nurses. Anim Behav 77:911–918
Nosil P (2002) Transition rates between specialization and generalization in phytophagous insects. Evolution 56:1701–1706
Novotny V, Basset Y, Miller SE, Weiblen GD, Bremer B, Cizek L, Drozd P (2002) Low host specificity of herbivorous insects in a tropical forest. Nature 416:841–844
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton, NJ
Passera L, Roncin E, Kaufmann B, Keller L (1996) Increased soldier production in ant colonies exposed to intraspecific competition. Nature 379:630–631
Patel AD (1990) An unusually broad behavioral repertory for a major worker in a dimorphic ant species: Pheidole morrisi (Hymenoptera: Formicidae). Psyche 97:181–192
Pie MR, Traniello JFA (2007) Morphological evolution in a hyperdiverse clade: the ant genus Pheidole. J Zool 271:99–109
Pitman NCA, Terborgh JW, Silman MR, PN V, Neill DA, Ceron CE, Palacios WA, Aulestia M (2001) Dominance and distribution of tree species in upper Amazonian terra firme forests. Ecology 82:2101–2117
Porter SD, Tschinkel WR (1985) Fire ant polymorphism: the ergonomics of brood production. Behav Ecol Sociobiol 16:323–336
Powell S, Franks NR (2006) Ecology and the evolution of worker morphological diversity: a comparative analysis with Eciton army ants. Funct Ecol 20:1105–1114
Rana JS, Dixon AFG, Jarosik V (2002) Costs and benefits of prey specialization in a generalist insect predator. J Anim Ecol 71:15–22
Rasband WS (2007) ImageJ. v 1.38. U.S. National Institutes of Health, Bethesda, MD
Rueffler C, Van Dooren TJM, Metz JAJ (2007) The interplay between behavior and morphology in the evolutionary dynamics of resource specialization. Am Nat 169:E34–E52
Safranek L, Williams CM (1984) Determinants of larval molt initiation in the tobacco hornworm, Manduca sexta. Biol Bull 167:568–578
Schmid-Hempel P (1992) Worker castes and adaptive demography. J Evol Biol 5:1–12
Schneirla TC (1971) Army ants: a study in social organization. Freeman, San Francisco, CA
Seid MA, Goode K, Li C, Traniello JFA (2008) Age-and subcaste-related patterns of serotonergic immunoreactivity in the optic lobes of the ant. Dev Neurobiol 68:1325–1333
Seid MA, Traniello JFA (2005) Age-related changes in biogenic amines in individual brains of the ant Pheidole dentata. Naturwissenschaften 92:198–201
Sempo G, Detrain C (2004) Between-species differences of behavioural repertoire of castes in the ant genus Pheidole: a methodological artefact? Insectes Soc 51:48–54
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75:2–16
Timms R, Read AF (1999) What makes a specialist special? Trends Ecol Evol 14:333–334
Topoff H (1971) Polymorphism in army ants related to division of labor and colony cyclic behavior. Am Nat 105:529–548
Tschinkel WR (2006) The fire ants. Harvard University Press, Cambridge, MA
Wcislo WT, Cane JH (1996) Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stored foods by natural enemies. Annu Rev Entomol 41:257–286
Weber NA (1972) Gardening ants: the attines. American Philosophical Society, Philadelphia, PA
Welch BL (1951) On the comparison of several mean values: an alternative approach. Biometrika 38:330–336
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, New York, NY
Wetterer JK (1995) Forager size and ecology of Acromyrmex coronatus and other leaf-cutting ants in Costa Rica. Oecologia 104:409–415
Wheeler DE (1991) The developmental basis of worker caste polymorphism in ants. Am Nat 138:1218–1238
Wheeler DE, Nijhout HF (1984) Soldier determination in Pheidole bicarinata: inhibition by adult soldiers. J Insect Physiol 30:127–135
Wilson EO (1971) The insect societies. Harvard University Press, Cambridge, MA
Wilson EO (1976) The organization of colony defense in the ant Pheidole dentata Mayr (Hymenoptera: Formicidae). Behav Ecol Sociobiol 1:63–81
Wilson EO (1978) Division of labor in fire ants based on physical castes (Hymenoptera: Formicidae: Solenopsis). J Kans Entomol Soc 51:615–636
Wilson EO (1980) Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). Behav Ecol Sociobiol 7:143–156
Wilson EO (1983) Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). III: ergonomic resiliency in foraging by Atta cephalotes. Behav Ecol Sociobiol 14:47–54
Wilson EO (1984) The relation between caste ratios and division of labor in the ant genus Pheidole (Hymenoptera: Formicidae). Behav Ecol Sociobiol 16:89–98
Wilson EO (1985) Between-caste aversion as a basis for division of labor in the ant Pheidole pubiventris (Hymenoptera: Formicidae). Behav Ecol Sociobiol 17:35–37
Wilson EO (2003) Pheidole in the new world: a dominant, hyperdiverse ant genus. Harvard University Press, Cambridge, MA
Yang AS, Martin CH, Nijhout HF (2004) Geographic variation of caste structure among ant populations. Curr Biol 14:514–519
Acknowledgments
We thank the directors and staff of Tiputini Biodiversity Station for their assistance in obtaining permits and support in the field. We thank Clifton Meek, Megan Johnson, Brian Henry, Noah Reid, Scott Appleby, Elise Koncsek, Frank Azorsa Salazar, Wendy Mertl, and Winston McDonald for field and laboratory assistance. We are very grateful to Stefan Cover for confirming Pheidole identifications, to Dr. Gary Alpert for instruction on digital imaging, to Dr. Marcio Pie for suggestions on morphometric analyses, and to Dr. Marc Seid for assistance developing ethogram techniques. We thank Dr. Michael Kaspari, Dr. Michael Sorenson, and two anonymous reviewers for constructive comments on the manuscript. This work was funded by a National Science Foundation Graduate Research Fellowship awarded to ALM. JT was funded by NSF Grants IOB 0725013 and 0724591 awarded to J. Traniello and W. Gronenberg. Voucher samples were collected and transported under permit 017-IC-FA-PNY-MA issued to ALM by the Ecuadorian Ministry of the Environment. This work complied with all current laws of Ecuador and the USA. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Rueppell
Dedicated to Professor Edward O. Wilson on the occasion of his 80th birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
List of tasks observed during full colony ethograms. Tasks are categorized as brood-care and nonbrood-care tasks (PDF 9 kb)
Table S2
Number of brood-care acts observed during intact colony ethograms and monocaste subcolony observations. Means (standard deviations) of total acts observed per colony are shown; values are not corrected for number of ants, number of brood, or observation time (PDF 15 kb)
Table S3
Proportion of brood of various developmental stages surviving in monocaste subcolonies. Z and P values represent Wilcoxon sign rank tests comparing the survival of brood cared for by major workers to those cared for by minor workers from the same colony after 1 and 2 weeks. n number of colonies (Table 1) (PDF 13 kb)
Table S4
Eigenvectors for the first three principal components in an analysis of an unrotated correlation matrix of Z scores for seven variables, which represent the degree of morphological difference between majors and minor for ten Pheidole species. These three principal components explained 85.1% of the total variance and were the only components with eigenvalues greater than one. PCA components were interpreted based on the morphological variables with strong loadings on each component. Loadings ≥0.49 or ≤ −0.49 were considered “strong” are and shown in bold (PDF 68 kb)
Rights and permissions
About this article
Cite this article
Mertl, A.L., Traniello, J.F.A. Behavioral evolution in the major worker subcaste of twig-nesting Pheidole (Hymenoptera: Formicidae): does morphological specialization influence task plasticity?. Behav Ecol Sociobiol 63, 1411–1426 (2009). https://doi.org/10.1007/s00265-009-0797-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0797-3