Abstract
The response rates of PD-1/PD-L1 blockade in cancer immunotherapy are relatively low, necessitating the development of novel immune checkpoint inhibitors. Compared with other immune checkpoints, VISTA interacts with its ligand PSGL-1 only under acidic conditions in the tumor microenvironment to suppress the function of CD8+ T cells. On the other hand, drug repurposing offers advantages such as time efficiency and high safety. However, the development of VISTA/PSGL-1 inhibitor based on drug repurposing is still infancy. Here, by screening a library of marketed drugs, we identified Chidamide had a strong binding affinity toward VISTA (KD = 5 nM) and blocked VISTA/PSGL-1 under acidic conditions, thereby significantly enhancing the function of CD8+ T cells and inhibiting the tumor growth in immunocompetent murine CT26 tumor model. This study represents the first discovery of Chidamide as VISTA/PSGL-1 blocker for cancer immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint blockade (ICB) therapy stands as a potent therapeutic strategy against cancer and achieves significant advancements in clinical oncology [1]. Particularly noteworthy are antibodies directed toward cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1), both integral components of the B7 family of immune checkpoint receptors, which have exhibited remarkable efficacy across a spectrum of malignancies [2, 3]. Despite these commendable accomplishments, the efficacy of ICB therapy remains modest, highlighting the potential for targeting additional non-redundant immunomodulatory pathways to amplify therapeutic outcomes while attenuating immunosuppressive mechanisms [4,5,6,7].
The influence of acidic conditions on molecular interactions, particularly the weakening of protein–protein binding, is well documented. This phenomenon extends beyond the confines of tumor microenvironments to encompass lymph nodes, which are recognized for their mildly acidic milieu. Hence, the investigation into the pivotal role of immune checkpoint molecules under acidic conditions assumes pivotal importance [8]. Disruption of the inhibitory interaction between immune checkpoint receptors and ligands under acidic conditions holds the potential to enhance T cell activation within lymph nodes and tumor microenvironment, thereby potentially ameliorating the immune-related adverse effects associated with conventional immune checkpoint inhibitors. V-domain Ig suppressor of T cell activation (VISTA) is noteworthy in this context, a notable member of the B7 family, implicated in exerting immunosuppressive functions within acidic tumor environments [9]. Different from its counterparts, VISTA’s extracellular domain harbors a distinctive histidine-rich region, facilitating conformational alterations under acidic conditions (pH 6.0) within the tumor microenvironment [10]. Consequently, this facilitates specific binding to its ligand PSGL-1 and subsequent suppression of T cell functionality [11].
VISTA is prominently expressed on diverse immune cell subsets, notably T cells, macrophages, and myeloid-derived suppressor cells (MDSCs) [12]. Disrupting the VISTA/PSGL-1 interaction under acidic microenvironments is integral to reinstating T cell functionality [13]. Despite the progression of antibodies targeting VISTA into clinical trials, the development of small molecule inhibitors encounters limitations [14,15,16]. Repurposing existing pharmacotherapeutic agents emerges as an attractive strategy, offering expedited developmental trajectories, augmented safety profiles, and alleviated financial burdens. Various agents have showcased efficacy in broadening therapeutic indications and unveiling novel mechanisms [17], thus providing robust rationale for clinical pharmacotherapy. Remarkably, the antihypertensive drug azenidipine has been identified for repurposing in cancer immunotherapy, demonstrating efficacy through the simultaneous inhibition of the CD47/SIRPα and TIGIT/PVR pathways [18]. Concurrently, the natural compound 3,5-diiodotyrosine is under investigation as an APOBEC3B inhibitor, aimed at mitigating somatic mutation accumulation and subsequent tumor progression [19]. Furthermore, recent studies revealed that Hemin offered significant advantages in tumor immunotherapy by disrupting the TIGIT/PVR interaction and enhancing ferroptosis in tumor cells [20].
In the present study, we carry out a comprehensive examination of the interaction interfaces between VISTA and PSGL-1, employing a rigorous analysis of commercially available drug libraries to pinpoint agents capable of impeding VISTA/PSGL-1 binding under acidic conditions of tumor microenvironment. Through a series of blocking assays, binding assays, and functional assays, we identify Chidamide as a potent blocker of VISTA/PSGL-1 interaction under acidic tumor conditions, thereby restoring T cell functionality. Chidamide, a FDA-approved small molecule anti-tumor agent starting its clinical trial in USA since 2010, further endorsed by the CFDA in 2014, which is predominantly utilized in the management of refractory peripheral T cell lymphoma [21]. The expansion of therapeutic indications for Chidamide, sanctioned in 2023, encompasses breast cancer therapy [22]. Operating through epigenetic regulatory pathways targeting HDACs, Chidamide facilitates the differentiation of tumor stem cells and mitigates epithelial–mesenchymal transition in tumor cells, potentially reinstating drug sensitivity in resistant tumor subpopulations and impeding tumor metastasis and recurrence [23, 24]. Our investigation presents, for the first time, the prospect of Chidamide in revitalizing T cell functionality via the disruption of VISTA/PSGL-1 binding within acidic tumor microenvironment, thereby potentially enhancing anti-tumor efficacy, particularly in combination with PD-1/PD-L1 blockade.
Materials and methods
Cell lines
Both CHO-K1-hVISTA and CHO-K1-hPD-L1 cells were constructed by lentivirus transfection. Murine-derived lymphocytes were obtained from spleens sites of 6-week-old BALB/c mice, specifically after three days of stimulation with 1 μg/mL CD3 and 0.5 μg/mL CD28 antibodies from a single-cell suspension of mouse spleen cells. Murine colon cell line CT26 was gifted by Prof. Shengdian Wang from Institute of Biophysics, Chinese Academy of Sciences. All cells were cultured in an incubator with 5% CO2 at 37 °C.
Mice
Female BALB/c mice, aged six weeks, were sourced from Zhuhai BesTest Bio-Tech Co., Ltd. and were housed in a specific pathogen-free facility. All animal experiments comply with the reach guidelines and were approved by the Ethics Committee of Sun Yat-sen University (approval number: SYSU-IACUC-2024-000515).
Virtual screening of small molecules targeting VISTA
The structure of VISTA (PDB ID: 6OIL) was retrieved from the Protein Data Bank (PDB) and subjected to structure preparation and energy minimization using the MOE software. The missing amino acids in the protein structure were restored through structural modeling with the “Loop Modeler” module in the MOE software. A commercially available drug library was processed by conducting washing, desalting, energy minimization, and 3D structure protonation in MOE. The prepared small molecule library was docked to the loop region consisting of H153, H154, and H155 of VISTA using the “Dock” module within the MOE software. The docking results were ranked based on the S-score, where a lower S-value indicates a more favorable interaction. Finally, a comprehensive analysis encompassing the S-score, amino acid interactions, binding models, and binding energies was performed to select 11 candidate small molecules for subsequent experimental validation.
Blocking assay
CHO-K1-hVISTA cells were adjusted at a density of 2 × 105 cells per tube. Following PBS washing, the supernatant was removed through centrifugation, and the small molecules were diluted to a concentration of 100 μM. The cells were then incubated with the small molecules (TargetMol, USA) or anti-PSGL-1 (10 μg/mL) (R&D Systems, 688102) at 4 °C for 30 min. After the incubation, 10 μL of 200 ng hPSGL-1-Fc protein (ACRO Biosystems China) was added and further incubated for 30 min at 4 °C. Finally, Fc-PE antibody was added and incubated at 4 °C for 30 min. The cells were washed with PBS, and the supernatant was removed after centrifugation. The cells were then resuspended in flow cytometry buffer. All incubation steps were performed at indicated pH (pH6.0 or pH7.2).
MST assay
The human VISTA-IgV domain protein fused with His tag (ACRO Biosystems, China) was labeled with Red-NHS647 dye and used for subsequent experiments. The small molecules were serially diluted in PBST buffer. The labeled protein was then incubated with small molecules at different concentrations. Subsequently, the samples were loaded into standard capillaries and analyzed using an MST instrument (Nano Temper, Monolith NT.115, Germany).
MTT assay
Single-cell suspensions of CT26 colon cancer cells were seeded at a density of 3,000 cells per well in a flat-bottomed 96-well plate and cultured overnight in the incubator. The cell medium was then replaced with 200 μL of fresh serum-free medium at 37 °C for 8 h. Subsequently, the small molecules at different concentrations were added. Control wells were treated with an equivalent volume of DMSO. The plate was further incubated for 24, 48, and 72 h, respectively. At each time point, 20 μL of MTT solution was added. After incubation for 4 h, the supernatant was discarded, and 150 μL of DMSO was added. The plate was thoroughly shaken for 10 min. Finally, the absorbance was measured at 490 nm.
Co-culture assay
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy individuals and seeded at a density of 2 × 105 cells per well in a 48-well plate (SORFA, China). The cells were stimulated with 10 μg/mL of phytohemagglutinin (PHA) at 37 °C for 4–6 h. The groups consisted of the following: unstimulated group (PBMCs), stimulated group (PBMCs + PHA), negative control group (PBMCs + PHA + CHO-K1 and PBMCs + PHA + CHO-K1-hVISTA), positive control group (PBMCs + PHA + CHO-K1-hVISTA + 10 μg/mL anti-PSGL-1), and experimental group (PBMCs + PHA + CHO-K1-hVISTA + 10 μM Chidamide), along with protein transport inhibitor (555029, BD Bioscience). After the incubation, the cells were collected for intracellular cytokine staining using the following antibodies: anti-human CD3 (SK7, eBioscience), anti-human CD8 (SK1, eBioscience), and anti-human IFN-γ (4S.B3, eBioscience). Normal IMDM medium and pH 6.0 IMDM medium were used in the experiment.
T cell memory experiment
Spleen cells from BALB/c mice were isolated, processed, and treated with 1 × ACK at room temperature for 10 min. Following centrifugation, the cells were resuspended in culture medium, stimulated with 1 μg/mL CD3 and CD28 antibodies, and supplemented with 100 U/mL IL-2 to maintain cell viability. After 3 days of stimulation, the cells were exposed to 10 μM Chidamide for 24 h. Subsequently, anti-mouse CD8 (53–6.7, eBioscience), anti-mouse CD44 (IM7, eBioscience), and anti-mouse CD62L (MEL-14, eBioscience) were used for staining, along with isotype controls for detection.
qPCR experiment
T cells were seeded at a density of 2 × 105 cells per well in a 96-well plate. The control group was treated with an equal volume of DMSO, while the treatment group received 10 μM Chidamide. And RNA was extracted using an RNA extraction kit. The extracted RNA was then reverse transcribed into cDNA using a reverse transcription kit. The qPCR was performed using the cDNA as a template, SYBR Green Master Mix as the enzyme for the reaction, and specific primers. The primer sequences used in the experiment are provided below.
m-Sell-F: 5’-TACATTGCCCAAAAGCCCTTAT-3’
m-Sell-R: 5’-CCTCCTTGGACTTCTTGTTGTT-3’
m-CCR7-F: 5’-CAGGTGTGCTTCTGCCAAGAT-3’
m-CCR7-R: 5’-GGTAGGTATCCGTCATGGTCT-3’
m-IL-7R-F: 5’-GCGGACGATCACTCCTTCTG-3’
m-IL-7R-R: 5’-AGCCCCACATATTTGAAATTCCA-3’
m-TCF7-F: 5’-CCACTCTACGAACATTTCAGCA-3’
m-TCF7-R: 5’-ACTGGGCCAGCTCACAGTA-3’
m-EOMES-F: 5’-GGCCCCTATGGCTCAAATTCC-3’
m-EOMES-R: 5’-GAACCACTTCCACGAAAACATTG-3’
m-TBX21-F: 5’-AGCAAGGACGGCGAATGTT-3’
m-TBX21-R: 5’-GTGGACATATAAGCGGTTCCC-3’
Co-culture of CT26 cells with T cells from CT26 tumor-bearing mice
Spleens and lymph nodes were excised from CT26 tumor-bearing mice, followed by mechanical dissociation and filtration to isolate single-cell suspensions. Red blood cells were lysed using a standard red blood cell lysis buffer. The resulting cells were stimulated with anti-mouse CD3 (1 μg/mL, Biolegend, 100340) and anti-mouse CD28 (1 μg/mL, Biolegend, 102116) for 48 h. Subsequently, CT26 cells and the activated T cells were co-cultured in a 48-well plate at a 1:10 ratio (CT26:T cells) for 6 h at 37 °C in a 5% CO₂ atmosphere. Two different culture media were used: normal pH7.4 medium and acidic pH6.0 medium, the latter adjusted using sulfonic acid. Following incubation, CT26 cells were collected for apoptosis analysis (YEASEN, 40130ES60), and T cells were harvested for flow cytometric evaluation of IFN-γ secretion by CD8+ T cells.
CT26 mouse model experiment
Female BALB/c mice, aged six weeks, were administered intraperitoneal injections of Chidamide or PD-1/PD-L1 blocking peptide OPBP-1 over a two-week period [25]. Tumor volume was calculated using the formula V = 1/2 × a(length) × b(width) × c(height), while the tumor suppression rate was determined as (average weight of control l group-average weight of experimental group) / average weight of control group × 100%. HE staining procedures were carried out by Wuhan servicebio Biotechnology Co., Ltd.
Ex vivo analysis of CD8 + T cells and MDSCs
The tumor tissue from tumor-bearing mice was immediately dissected. To detect the CD8+ T cells within the tumor, the tumor tissue was finely minced and incubated with collagenase IV (Invitrogen, USA) and DNase I (Sigma, USA) at 37 ℃ for 40 min. After digestion, the samples were filtered and tumor cells were collected after centrifugation. The collected tumor cells were then stained with anti-mouse CD45 (30-F11, eBioscience), anti-mouse CD3 (17A2, eBioscience), anti-mouse CD8α (53–6.7, eBioscience), or matched isotype control antibodies. Flow cytometry analysis was conducted for subsequent characterization. For the detection of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) within the tumor tissue, tumor cells were treated with anti-mouse CD45 (30-F11, eBioscience), anti-mouse CD11b (M1/70, eBioscience), anti-mouse Ly6G (1A8, Biolegend), and anti-mouse PD-L1 (MIH5, eBioscience).
Intracellular IFN-γ staining
Lymph nodes from the mice were excised and processed through grinding. Tumor-infiltrating lymphocytes (TILs) were isolated from the digested tumor tissues. The single-cell suspensions of TILs and draining lymph nodes were plated in a 24-well plate and stimulated with 20 ng/mL of phorbol 12-myristate 13-acetate (PMA, Sigma, USA) and 1 µM of ionomycin (Sigma, USA), along with the protein transport inhibitor (555029, BD Bioscience) for 4 h. Subsequently, the cells were stained with anti-mouse CD3 (17A2, eBioscience) and anti-mouse CD8α (53–6.7, eBioscience) at 4 °C for 30 min. Subsequently, the CD8+ T cells were fixed, permeabilized, and stained with anti-mouse IFN-γ (XMG1.2, eBioscience) to detect the production of IFN-γ using flow cytometry.
Staining of memory T cells in mouse spleens
Single-cell suspensions of the spleens of mice were stained with anti-mouse CD3 (17A2, eBioscience), anti-CD8 (53–6.7, eBioscience), anti-CD44 (IM7, eBioscience), and anti-CD62L (MEL-14, eBioscience), or isotype controls. Flow cytometric analysis was performed to determine the proportions of effector memory T cells (TEM) and central memory T cells (TCM).
Results
Virtual screening of small molecule inhibitors for VISTA, an immune checkpoint in the immune microenvironment
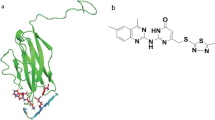
We obtained a uniformly standardized pan-cancer dataset from the UCSC database, comprising TCGA TARGET GTEx (PANCAN, N = 19,131, G = 60,499). From this dataset, we extracted the expression data of the VISTA (C10orf54) gene across various samples and calculated the immune scores for colorectal cancer (COAD) and breast cancer (BRCA) patients based on gene expression profiles. The analysis revealed a positive correlation between VISTA expression and immune infiltration scores in both colorectal and breast cancer patients (Fig. 1A, C). Furthermore, we investigated the association between VISTA expression and the expression levels of TIM-3 (HAVCR2), PD-1 (PDCD1), and LAG-3 (LAG3). Our findings demonstrated that VISTA expression was positively correlated with the expression of TIM-3, PD-1, and LAG-3 in both colorectal and breast cancer cohorts (Fig. 1B, D). These results underscore the pivotal role of VISTA as a unique immune checkpoint molecule within the tumor microenvironment. The extracellular domain of VISTA harbors multiple histidine residues (His153, His154, and His155), facilitating its binding to PSGL-1 under acidic conditions [10]. Selective disruption of this interaction under acidic conditions holds promise for reversing VISTA-mediated immune suppression [11, 27]. We retrieved the protein structure of VISTA from the Protein Data Bank (PDB ID: 6OIL). We began by optimizing the protein structure using the Molecular Operating Environment (MOE) and identifying regions rich in histidine residues as possible binding sites for small compounds (Fig. 1E, F). Subsequently, utilizing a minimally energy-optimized library of commercially available small molecule drugs, we performed molecular docking against the selected binding pocket of the VISTA protein. The resulting conformations were then ranked based on their respective scoring metrics (S-score), yielding 11 candidate small molecules for subsequent functional validation (Fig. 1E). At the cellular level, we initially evaluated the ability of candidate small molecules to disrupt VISTA/PSGL-1 interaction under acidic conditions (pH 6.0). Results demonstrated compounds 2, 3, 7, and 8 exhibited inhibitory effects on VISTA/PSGL-1 binding under acidic conditions (Fig. 1G).
Correlation of VISTA expression with immune response in cancer patients as well as virtual screening for VISTA/PSGL-1 inhibitors. A, C Using the UCSC (https://xenabrowser.net/) database, we retrieved a uniformly standardized pan-cancer dataset (TCGA TARGET GTEx, PANCAN, N = 19,131, G = 60,499). From this dataset, we extracted the expression data of the VISTA (C10orf54) gene across various samples. Immune scores for colorectal cancer (COAD) and breast cancer (BRCA) patients were subsequently computed based on gene expression. B, D The correlation between VISTA expression and the expression levels of TIM-3, PD-1, and LAG-3 in colorectal cancer (COAD) and breast cancer (BRCA) patients was analyzed using the Timer website (http://timer.cistrome.org/). E Schematic representation of the small molecule screening process. Virtual screening focused on the histidine-rich loop region of VISTA, leading to the selection of the top 11 small molecules for further analysis. F Identification of key histidine residues H153, H154, and H155, crucial for the interaction between VISTA and PSGL-1. The hVISTA structure was depicted in yellow, with an emphasis placed on critical histidine residues shown in pink sticks. G Assessment of the efficacy of the 11 candidate small molecules (100 μM) and anti-PSGL-1 (10 μg/mL) in inhibiting VISTA/PSGL-1 binding under pH 6.0 conditions. Consistent outcomes were observed across three independent experiments. Data are presented as means ± SEM. Error bars depict the standard deviation of the three independent experiments
Binding affinity of Chidamide to VISTA
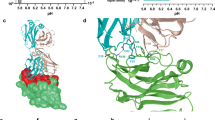
Since VISTA and PD-1 share sequence and structure similarities [26, 27], in order to investigate whether potential small compounds can similarly prevent PD-1/PD-L1 from binding, we systematically investigated the disruptive potential of compounds 2, 3, 7, and 8 on the PD-1/PD-L1 interaction across both acidic and neutral conditions. There is no difference between the affinity of PD-1/PD-L1 at both pH 6.0 and pH 7.2 (Fig. 2A), and neither the compounds 2, 3, 7, and 8 exhibited any interference of the PD-1/PD-L1 blockade under either condition (Fig. 2B, C), which indicating the specific binding to VISTA. Subsequent microscale thermophoresis (MST) assays revealed compound 7, Chidamide, to exhibit remarkable affinity to VISTA, boasting a KD value of 5 nM (Fig. 2D). Furthermore, by exploiting the structural similarity of Chidamide, we screened the clinically approved compounds and identified Mocetinostat shared the highest structural resemblance (Fig. 2E). Blocking assays were performed to underscore the efficacy of Mocetinostat in abrogating the VISTA/PSGL-1 interaction, and Mocetinostat exhibits a similar blocking efficacy to Chidamide (Fig. 2F). Finally, by employing MOE for molecular docking, we delineated the putative interaction model between Chidamide and VISTA. The results indicated that Chidamide engaged in hydrogen bonding with His154 and His155, thereby effectively occupied the histidine-rich loop region within the VISTA protein (Fig. 2G).
Specific inhibition of VISTA/PSGL-1 interaction by Chidamide. A Affinity between PD-1 and PD-L1 under both acidic and neutral conditions. B Inhibitory efficacy of four candidate small molecules on PD-1/PD-L1 interactions under acidic conditions; anti-PD-1 was used as a positive control. C Inhibitory efficacy of four candidate small molecules on PD-1/PD-L1 interactions under neutral conditions; anti-PD-1 was used as a positive control. D Affinity of the four candidate small molecules for the VISTA protein. E, F Structural depiction (E) and inhibitory efficacy (F) of Chidamide and its analogs on VISTA/PSGL-1 interaction. G Interaction mode between Chidamide and the hVISTA protein. Chidamide was depicted as pink sticks. The His154 and His155 residues of hVISTA were represented as cyan sticks, and hydrogen bonds between hVISTA and Chidamide were denoted by black dashed lines. Similar results were replicated across three independent experiments. Data are presented as means ± SEM. Error bars depict the standard deviation from three independent experiments
Chidamide restores VISTA/PSGL-1-mediated suppression of CD8 + T cells under acidic conditions
Studies have indicated that blocking the interaction between VISTA and PSGL-1 effectively restored the functionality of CD8+ T cells under acidic conditions [28]. Lymphocytes isolated from the peripheral blood of healthy donors were stimulated with PHA under both acidic and neutral conditions. Flow cytometric analysis revealed that VISTA and its ligand PSGL-1 were highly expressed on the surface of CD3+ T cells (Fig. 3A, C). Following stimulation and treatment with Chidamide under acidic conditions, both VISTA and PSGL-1 remained highly expressed on the surface of CD3+ T cells, with enhanced IFN-γ secretion observed from CD8+ T cells (Fig. 3B). However, when cells were treated under neutral conditions, no significant increase in IFN-γ secretion by CD8+ T cells was noted compared to the stimulation-only group (Fig. 3D), indicating that Chidamide’s effect is restricted to acidic conditions. To further investigate the relationship between enhanced CD8+ T cell function and the VISTA/PSGL-1 interaction, PBMCs from healthy donors were co-cultured with CHO-K1 cells overexpressing human VISTA (CHO-K1-hVISTA) under both acidic and neutral conditions (Fig. 3F, I). CHO-K1 cells served as negative controls (Fig. 3C). High expression of VISTA and PSGL-1 on CD3+ T cells derived from PBMCs was confirmed (Fig. 3E, H). Co-culture with CHO-K1 cells did not affect CD8+ T cell function under acidic conditions. In contrast, co-culture with CHO-K1-hVISTA cells inhibited IFN-γ secretion by CD8+ T cells, an effect that was reversed by Chidamide, leading to enhanced IFN-γ secretion (Fig. 3G). This effect was absent under neutral conditions (Fig. 3J). These results suggested that Chidamide enhanced T cell function by specifically blocking the VISTA/PSGL-1 interaction under acidic conditions.
Chidamide alleviated VISTA/PSGL-1-mediated immunosuppression and enhances IFN-γ secretion by CD8+ T cells. A, C Expression levels of VISTA and PSGL-1 on the surface of CD3+ T cells derived from PBMCs under acidic and neutral culture conditions. B, D PBMCs were isolated from healthy donors and stimulated for three days. The cells were then treated with Chidamide for 6 h in either normal pH medium (pH 7.4) or acidic medium (pH 6.0). Dimethyl sulfoxide (DMSO) served as a negative control, while anti-PSGL-1 was used as a positive control. Intracellular cytokine staining was performed to assess IFN-γ secretion by CD8+ T cells via flow cytometry. E, H Expression levels of VISTA and PSGL-1 on the surface of CD3+ T cells derived from PBMCs under acidic and neutral culture conditions in co-culture system. F, I Schematic illustration of the co-culture experiment. G, J Following PBMC isolation and a three-day stimulation from healthy donors, the cells were co-cultured with either CHO-K1-hVISTA or VISTA-negative CHO-K1 cells. Chidamide treatment was administered for 6 h at pH 6.0 and pH 7.4, with DMSO as the negative control and anti-PSGL-1 as the positive control. Intracellular cytokine staining and flow cytometry were subsequently conducted to measure IFN-γ secretion by CD8+ T cells. The combined data are depicted as means ± SEM and analyzed utilizing one-way ANOVA. Significance levels were indicated as * (p < 0.05) and ** (p < 0.01) based on the results of one-way ANOVA
Chidamide suppresses CD8 + T cell proliferation but enhances memory capacity
We further evaluated the impact of Chidamide on CD8+ T cell proliferation. Interestingly, we observed a concentration-dependent inhibition of CD8+ T cell proliferation with increasing concentrations of Chidamide (Fig. 4A, B). This finding aligns with previous reports demonstrating the influence of Chidamide on T cell proliferation in the treatment of lymphoma [21, 29, 30]. Subsequently, under acidic conditions, murine splenocytes were stimulated and treated with Chidamide. The results revealed that Chidamide enhanced the secretion of IFN-γ by CD8+ T cells under acidic conditions (Fig. 4C, D). Subsequently, by employing flow cytometry, we assessed the influence of Chidamide on the expression of CD44 and CD62L in CD8+ T cells. Our results revealed that Chidamide increased the proportion of CD44hi CD62Lhi cells, indicative of the ability of Chidamide to promote the transition of CD8+ T cells toward central memory T cells (TCM) (Fig. 4E, F), although the cluster of T cell subpopulation is not very well [31]. Furthermore, we examined the alterations in gene expression associated with T cell memory. Quantitative PCR (qPCR) analysis demonstrated a significant upregulation of CCR7, IL-7R, TCF-7, and TBX21 expression in T cells following 24 h treatment of Chidamide, genes closely associated with TCM formation (Fig. 4G).
Chidamide inhibited CD8+ T cell proliferation while enhancing their function. A, B Effects of varying concentrations of Chidamide on CD8+ T cell proliferation. C, D Spleen T cells from BALB/c mice were stimulated for three days and then treated with Chidamide for 6 h at pH 6.0, with DMSO as the control. Intracellular cytokine staining and flow cytometry were used to measure IFN-γ secretion by CD8+ T cells. E, F CD8+ T cells were treated with 10 μM Chidamide for 24 h, followed by flow cytometry analysis to detect the expression of CD44 and CD62L. (G) CD8+ T cells were treated with 10 μM Chidamide for 24 h, and qPCR was performed to assess the impact of Chidamide on the expression of T cell memory-related factors. The combined data are presented as means ± SEM and analyzed using one-way ANOVA. Significance levels were denoted as * (p < 0.05) and *** (p < 0.001) based on the outcomes of one-way ANOVA. “n.s.” represents no significance
When combined with anti-PD-1/PD-L1 peptide, Chidamide can boost its anti-tumor effects
Previous results highlighted the efficacy of Chidamide in restoring CD8+ T cell function and promoting tumor cell apoptosis [32]. To more precisely determine whether these effects are due to the blockade of VISTA interaction or the direct anti-tumor effects of Chidamide, we conducted a co-culture experiment using T cells and tumor cells in vitro. Specifically, spleen and lymph node cells from mice bearing CT26 tumors were stimulated and co-cultured with CT26 cells under both acidic and neutral conditions. The effects of Chidamide and the PD-1/PD-L1 blocking peptide OPBP-1 on tumor cell apoptosis and T cell function were assessed via flow cytometry. The results demonstrated that treatment with Chidamide did not influence the apoptosis of CT26 cells under either acidic or neutral conditions (Fig. 5A). However, Chidamide enhanced IFN-γ secretion by CD8+ T cells exclusively under acidic conditions (Fig. 5B). These findings suggested that Chidamide may enhance CD8+ T cell function in acidic conditions not through its anti-tumor effects, but by blocking the VISTA/PSGL-1 interaction. We further investigated its effects on tumor cells. MTT assays showed that Chidamide inhibited the proliferation of CT26 cells at concentrations above 10 μM after 48 and 72 h of treatment. Subsequently, we selected a concentration of 10 μM, which did not inhibit the proliferation of CT26 cell within 24 h for further exploration (Fig. 5C). We then assessed the impact of Chidamide on PD-L1 expression on the surface of CT26 cells. Results indicated that PD-L1 expression increased with higher Chidamide concentrations (Fig. 5D), suggesting a potential synergistic application of Chidamide and anti-PD-L1 drugs in treatment of colorectal cancer. To evaluate the anti-tumor effects of Chidamide alone and in combination with anti-PD-L1 therapy, we established a CT26 mouse colon cancer model. When tumor volumes reached 40–60 mm3, mice were administered 5 mg/kg of Chidamide intraperitoneally, or a combination of 5 mg/kg Chidamide and 0.5 mg/kg of the PD-L1 blockade peptide OPBP-1 (Fig. 5E). Tumor volumes and mouse weights were recorded every two days during treatment. Two weeks later, mice were euthanized, tumors were dissected and weighed, and heart, liver, spleen, lungs, and kidneys were subjected to H&E staining. The results showed that Chidamide significantly inhibited tumor growth, and the combination with OPBP-1 produced a more pronounced therapeutic effect (Fig. 5F–H). Additionally, there were no significant changes in mouse weight during the treatment (Fig. 5I), and H&E staining revealed no organ damage in either the Chidamide-treated or combination groups, demonstrating the safety and efficacy of this treatment approach (Fig. 5J).
Chidamide profoundly suppressed tumor growth in CT26 tumor-bearing mice, with augmented efficacy in combination with PD-1/PD-L1 blocking peptide OPBP-1. A Effects of Chidamide and OPBP-1 on CT26 cell apoptosis following co-culture with T cells derived from the spleen and lymph nodes of CT26 tumor-bearing mice, under both acidic and neutral conditions. B Effects of Chidamide and OPBP-1 on IFN-γ secretion by CD8+ T cells after co-culture with CT26 cells from the spleen and lymph nodes of CT26 tumor-bearing mice, under acidic and neutral conditions. C MTT assay to assess the impact of different concentrations of Chidamide on CT26 cell proliferation. D Flow cytometric analysis of PD-L1 expression on CT26 cells following a 24 h treatment with varying concentrations of Chidamide. E Schematic illustration of the treatment protocol in the CT26 tumor-bearing mouse model. F, G Tumors were harvested post-treatment, photographed, and weighed to determine tumor mass. H, I Tumor growth and body weight curves of mice throughout the treatment period. J Following treatment, major organs (heart, liver, spleen, lungs, and kidneys) were excised, fixed, embedded, and subjected to H&E staining. Photomicrographs were captured under a 10 × microscope. The data are presented as means ± SEM, with a sample size of n = 5. Statistical analysis was performed using one-way ANOVA, indicating significance levels as * (p < 0.05), ** (p < 0.01), and *** (p < 0.001). “n.s.” represents no significance
The anti-tumor properties of Chidamide are associated with the activation of immune response
Firstly, we analyzed the function of CD8+ T cells in the tumor site. The results indicated that Chidamide significantly enhanced the ability of CD8+ T cells to secrete IFN-γ within the tumor (Fig. 6A, B). Previous studies have reported that blocking VISTA can restore CD8+ T cell function and reduce the proportion of MDSCs [15, 35]. We further analyzed the changes of MDSCs at the tumor site. The results showed that both Chidamide and OPBP-1 alone reduced the proportions of M-MDSCs and PMN-MDSCs at the tumor site, with the combination treatment producing a greater reduction (Fig. 6C–E). To delve into the reasons behind the diminishing proportions of M-MDSCs and PMN-MDSCs, we conducted a thorough examination of PD-L1 expression levels on these cell types. The results indicated that all three treatment groups significantly increased PD-L1 expression on M-MDSCs and PMN-MDSCs (Fig. 6F–I). Subsequently, we analyzed the function of CD8+ T cells in the spleen. The results indicated that only the OPBP-1 significantly increased IFN-γ secretion by CD8+ T cells from the spleen, while both Chidamide and the combination treatment significantly reduced IFN-γ secretion (Fig. 6J, K); this suggests that Chidamide may be more inclined to restore T cell function under acidic conditions. Additionally, we examined the changes in the proportions of TCM and TEM of CD8+ T cells in the spleen. The results showed that both Chidamide and the combination treatment significantly increased the proportion of TCM phenotype in splenic CD8+ T cells, while only the Chidamide treatment group significantly increased the proportion of the TEM phenotype (Fig. 6L–N), indicating that Chidamide promotes the differentiation of peripheral CD8+ T cells into TCM and TEM phenotypes. In summary, our in-depth investigation of the anti-tumor immune mechanism of Chidamide combined with PD-1/PD-L1 blockade demonstrated that Chidamide primarily exert strong anti-tumor effects by activating CD8+ T cells and reducing the infiltration of MDSCs at the tumor site, promoting the differentiation of peripheral splenic CD8+ T cells into memory phenotypes, and reducing peripheral immune side effects.
Chidamide demonstrated robust anti-tumor effects by enhancing CD8+ T cell function and reducing MDSC infiltration. A, B Lymphocytes from mouse tumors were isolated using Percoll and then stimulated with PMA and ionomycin for 4 h. Intracellular cytokine staining was performed to assess IFN-γ secretion by CD8+ T cells. C, D, E Flow cytometric analysis of M-MDSCs (Ly6GlowLy6Chigh) and PMN-MDSCs (Ly6GhighLy6Clow) infiltration at the tumor site. F, G Evaluation of PD-L1 expression on the surface of M-MDSCs at the tumor site. H, I Evaluation of PD-L1 expression on the surface of PMN-MDSCs at the tumor site. J, K Spleen cells were ground, lysed, and stimulated with PMA and ionomycin for 4 h. Intracellular cytokine staining was conducted to measure IFN-γ secretion by CD8+ T cells. L, M, N Analysis of TCM and TEM phenotypic alterations in CD8+ T cells in the spleen. The data are presented as means ± SEM, with a sample size of n = 5. Statistical analysis was performed using one-way ANOVA, indicating significance levels as follows: * (p < 0.05), ** (p < 0.01), and *** (p < 0.001)
Discussion
VISTA, also known as PD-1H, shares homology with PD-1 and serves as a significant immune checkpoint within the B7 family [33]. Unlike other immune checkpoints, the extracellular domain of VISTA contains a histidine-rich region that enables specific binding to its ligand PSGL-1, thereby inhibiting T cell function [10]. As a recently discovered immune checkpoint molecule, VISTA holds considerable potential for drug development. Specific antibodies targeting VISTA effectively inhibited the tumor growth in various murine tumor models, and their combination with PD-1/PD-L1 antibodies further enhanced the suppression [34,35,36,37]. In summary, VISTA emerges as a promising target for cancer immunotherapy. Drug development typically entails a protracted process; nonetheless, repurposing existing drugs offers several advantages [17, 38]. Thus, conducting virtual screening of small molecule drugs targeting the histidine-rich region of VISTA presents significant advantages. Through virtual screening, we identified Chidamide had a high affinity to VISTA. Notably, the histidine-rich region of VISTA contributes significantly to this interaction, potentially facilitating the binding of Chidamide to VISTA in the acidic tumor microenvironment, thereby disrupting its binding to PSGL-1 and restoring T cell function.
Histone deacetylases (HDACs) represent one of the most characteristicenzymes for epigenetic modifications, playing crucial roles in chromatin modification, gene expression regulation, and cell cycle differentiation and apoptosis [39, 40]. Dysregulation of epigenetic regulation can lead to the development and progression of cancer [41, 42]. Therefore, inhibitors targeting HDACs demonstrate significant clinical efficacy across multiple tumor types. Chidamide is the first orally available subtype-selective HDAC inhibitor (HDACi) drug approved in China, which is primarily used to treat relapsed or refractory peripheral T-cell lymphoma patients. Although the indications for HDACi are primarily focused on hematologic diseases, its role in cancer suggests potential applications across various tumor types [43]. Chidamide belongs to subtype-selective histone deacetylase inhibitors with minimal toxic side effects on normal cells. Therefore, in-depth research on its anti-tumor mechanisms can lay the foundation for drug development and provide evidence for the clinical treatment of malignant tumors. Our study found that Chidamide, as a VISTA/PSGL-1 blocker, enhanced the infiltration and activation of CD8+ T cells, reduced MDSCs infiltration at tumor sites, and increased the proportion of peripheral CD8+ T cells with central memory phenotype, thus exerting anti-tumor effects in the acidic tumor immune microenvironment.
In recent years, numerous studies have shown that Chidamide exhibited synergistic potential with other cancer treatment modalities, enhancing tumor suppression and even reversing resistance to other drugs, thereby improving treatment outcomes [44,45,46,47]. In this study, our results showed that Chidamide upregulated the expression of PD-L1 on the surface of tumor cells, which may be related to the involvement of STAT1 in the increased expression of PD-L1 induced by acetylation. Chidamide significantly reduced the infiltration of MDSCs at tumor sites, which might be associated with the upregulation of PD-L1 on the surface of MDSCs after treatment. Combination therapy of Chidamide with PD-1/PD-L1 blockade peptide OPBP-1 exhibited better therapeutic effects in a murine colon cancer model. Considering the advantages of peptide over antibody, development of peptide–drug conjugates comprising HDAC inhibitors and PD-1/PD-L1 blockade peptides warrants exploration for cancer immunotherapy.
Researchers have combined Chidamide with PD-1 antibodies and VEGF antibodies for the treatment of MSS/pMMR advanced colorectal cancer patients. The results of a Phase II clinical trial in 2024 indicated that the combination therapy enhanced the infiltration of CD8+ T cells, leading to a more immunologically active tumor microenvironment, suggesting a promising treatment option for MSS/pMMR advanced colorectal cancer [48]. However, the specific mechanisms underlying this combination therapy require further investigation. Our study found that Chidamide, as a VISTA/PSGL-1 blocker, enhanced the infiltration and activation of CD8+ T cells, reduced MDSCs infiltration at tumor sites, and increased the proportion of peripheral CD8+ T cells with central memory phenotype in the acidic tumor immune microenvironment. This may partially explain why Chidamide, in combination with PD-1 antibodies and VEGF antibodies, creates a more immunologically active tumor microenvironment in MSS/pMMR advanced colorectal cancer patients. In conclusion, our study elucidates potential mechanisms underlying the anti-tumor effects of Chidamide in colorectal cancer.
Despite its promise as an anti-lymphoma agent, Chidamide also presents certain limitations. While it has shown significant efficacy in recent years, especially in solid tumors, its impact on T cell proliferation remains a notable constraint. This limitation may explain its failure to induce regression in CT26 tumors, despite its enhancement of CD8+ T cell function via VISTA/PSGL-1 blockade. The therapeutic potential for rechallenged solid tumors remains largely unexplored, underscoring the need for further investigation into more effective combination therapies. Future research should focus on identifying synergistic strategies that can overcome these limitations, ultimately maximizing the therapeutic potential of Chidamide in cancer treatment.
Conclusions
In the acidic tumor microenvironment, Chidamide may bind to the unique histidine-rich region of VISTA and blocked VISTA/PSGL-1 interaction, thereby restoring the function of CD8+ T cells and inhibiting the tumor growth. Moreover, combined treatment with a PD-1/PD-L1 blockade peptide demonstrated enhanced anti-tumor efficacy. In summary, we elucidated an alternative potential mechanism by which Chidamide exerted its anti-tumor effects for cancer immunotherapy.
Data availability
No datasets were generated or analyzed during the current study.
References
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy[J]. J Clin Oncol 33(17):1974–1982
Curran MA, Montalvo W, Yagita H et al (2010) PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors[J]. Proc Natl Acad Sci U S A 107(9):4275–4280
Darvin P, Toor SM, Sasidharan Nair V et al (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers[J]. Exp Mol Med 50(12):1–11
Li B, Chan HL, Chen P (2019) Immune checkpoint inhibitors: basics and challenges[J]. Curr Med Chem 26(17):3009–3025
Rui R, Zhou L, He S (2023) Cancer immunotherapies: advances and bottlenecks[J]. Front Immunol 14:1212476
Deng J, Le Mercier I, Kuta A et al (2016) A new VISTA on combination therapy for negative checkpoint regulator blockade[J]. J Immunother Cancer 4:86
Sui X, Niu X, Zhou X et al (2023) Peptide drugs: a new direction in cancer immunotherapy[J]. Cancer Biol Med 21(3):198–203
Wu H, Estrella V, Beatty M et al (2020) T-cells produce acidic niches in lymph nodes to suppress their own effector functions[J]. Nat Commun 11(1):4113
Qin S, Xu L, Yi M et al (2019) Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4[J]. Mol Cancer 18(1):155
Johnston RJ, Su LJ, Pinckney J et al (2019) VISTA is an acidic pH-selective ligand for PSGL-1[J]. Nature 574(7779):565–570
Mehta N, Maddineni S, Kelly RL et al (2020) An engineered antibody binds a distinct epitope and is a potent inhibitor of murine and human VISTA[J]. Sci Rep 10(1):15171
Martin AS, Molloy M, Ugolkov A et al (2023) VISTA expression and patient selection for immune-based anticancer therapy. Front Immunol 20(14):1086102
Shekari N, Shanehbandi D, Kazemi T et al (2023) VISTA and its ligands: the next generation of promising therapeutic targets in immunotherapy[J]. Cancer Cell Int 23(1):265
Im E, Sim DY, Lee HJ et al (2022) Immune functions as a ligand or a receptor, cancer prognosis potential, clinical implication of VISTA in cancer immunotherapy[J]. Semin Cancer Biol 86:1066–1075
Zheng S, Zhang K, Zhang X et al (2022) Development of inhibitors targeting the V-domain Ig suppressor of T cell activation signal pathway[J]. J Med Chem 65(18):11900–11912
Sasikumar PG, Sudarshan NS, Adurthi S et al (2021) PD-1 derived CA-170 is an oral immune checkpoint inhibitor that exhibits preclinical anti-tumor efficacy[J]. Commun Biol 4(1):699
Pan J, Chen Y, Zhang Q et al (2021) Inhibition of lung tumorigenesis by a small molecule CA170 targeting the immune checkpoint protein VISTA[J]. Commun Biol 4(1):906
Zhou X, Jiao L, Qian Y et al (2021) Repositioning azelnidipine as a dual inhibitor targeting CD47/SIRPα and TIGIT/PVR pathways for cancer immuno-therapy[J]. Biomolecules. 11(5):706
Chen C, Sui X, Ning H et al (2022) Identification of natural product 3, 5-diiodotyrosine as APOBEC3B inhibitor to prevent somatic mutation accumulation and cancer progression[J]. J Immunother Cancer 11(5):706
Zhou X, Li Y, Zhang X et al (2024) Hemin blocks TIGIT/PVR interaction and induces ferroptosis to elicit synergistic effects of cancer immunotherapy[J]. Sci China Life Sci. 67(5):996–1009
Shi Y, Jia B, Xu W et al (2017) Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China[J]. J Hematol Oncol 10(1):69
Meng Y, Jin J, Gong C et al (2021) Phase II study of chidamide in combination with cisplatin in patients with metastatic triple-negative breast cancer[J]. Ann Palliat Med 10(11):11255–11264
Zhang P, Du Y, Bai H et al (2022) Optimized dose selective HDAC inhibitor tucidinostat overcomes anti-PD-L1 antibody resistance in experimental solid tumors[J]. BMC Med 20(1):435
Xue K, Wu JC, Li XY et al (2021) Chidamide triggers BTG1-mediated autophagy and reverses the chemotherapy resistance in the relapsed/refractory B-cell lymphoma[J]. Cell Death Dis 12(10):900
Li W, Zhu X, Zhou X et al (2021) An orally available PD-1/PD-L1 blocking peptide OPBP-1-loaded trimethyl chitosan hydrogel for cancer immunotherapy[J]. J Control Release 334:376–388
Niu X, Wu M, Li G et al (2023) Identification and optimization of peptide inhibitors to block VISTA/PSGL-1 interaction for cancer immunotherapy[J]. Acta Pharm Sin B 13(11):4511–4522
Yuan L, Tatineni J, Mahoney KM et al (2021) VISTA: a mediator of quiescence and a promising target in cancer immunotherapy[J]. Trends Immunol 42(3):209–227
Huang S, Zhao Y, Liao P et al (2022) Different expression patterns of VISTA concurrent with PD-1, Tim-3, and TIGIT on T cell subsets in peripheral blood and bone marrow from patients with multiple myeloma[J]. Front Oncol 10(12):1014904
Schaafsma E, Croteau W, ElTanbouly M et al (2023) VISTA targeting of T-cell quiescence and myeloid suppression overcomes adaptive resistance[J]. Cancer Immunol Res 11(1):38–55
Tonozuka Y, Tanaka H, Nomura K et al (2024) The combination of brentuximab vedotin and Chidamide synergistically suppresses the proliferation of T-cell lymphoma cells through the enhancement of apoptosis[J]. Cancer Chemother Pharmacol 93(2):137–149
Lv H, Lv G, Chen C et al (2020) NAD+ metabolism maintains inducible PD-L1 expression to drive tumor immune evasion[J]. Cell Metab 33(1):110–127
Gong C, Wu J, Song W et al (2023) Enhanced efficacy of combined fluzoparib and Chidamide targeting in natural killer/T-cell lymphoma[J]. Ann Hematol 102(10):2845–2855
Wang L, Jia B, Claxton DF et al (2018) VISTA is highly expressed on MDSCs and mediates an inhibition of T cell response in patients with AML[J]. Oncoimmunology 7(9):e1469594
Wang L, Rubinstein R, Lines JL et al (2011) VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses[J]. J Exp Med 208(3):577–592
Thakkar D, Paliwal S, Dharmadhikari B et al (2022) Rationally targeted anti-VISTA antibody that blockades the C-C’ loop region can reverse VISTA immune suppression and remodel the immune microenvironment to potently inhibit tumor growth in an Fc independent manner[J]. J Immunother Cancer 10(2):e003382
Iadonato S, Ovechkina Y, Lustig K et al (2023) A highly potent anti-VISTA antibody KVA12123 - a new immune checkpoint inhibitor and a promising therapy against poorly immunogenic tumors[J]. Front Immunol 12(14):1311658
Thisted T, Smith FD, Mukherjee A et al (2024) VISTA checkpoint inhibition by pH-selective antibody SNS-101 with optimized safety and pharmacokinetic profiles enhances PD-1 response[J]. Nat Commun 15(1):2917
Ashburn TT, Thor KB (2004) Drug repositioning: identifying and developing new uses for existing drugs[J]. Nat Rev Drug Discov 3(8):673–683
Chen R, Zhang M, Zhou Y et al (2020) The application of histone deacetylases inhibitors in glioblastoma[J]. J Exp Clin Cancer Res 39(1):138
Bhandari DR, Seo KW, Jung JW et al (2011) The regulatory role of c-MYC on HDAC2 and PcG expression in human multipotent stem cells[J]. J Cell Mol Med 15(7):1603–1614
Hogg SJ, Beavis PA, Dawson MA et al (2020) Targeting the epigenetic regulation of antitumour immunity[J]. Nat Rev Drug Discov 19(11):776–800
Davies A, Zoubeidi A, Beltran H et al (2023) The transcriptional and epigenetic landscape of cancer cell lineage plasticity[J]. Cancer Discov 13(8):1771–1788
Rai S, Kim WS, Ando K et al (2023) Oral HDAC inhibitor tucidinostat in patients with relapsed or refractory peripheral T-cell lymphoma: phase IIb results[J]. Haematologica 108(3):811–821
Li X, Yuan X, Wang Z et al (2022) Chidamide reverses fluzoparib resistance in triple-negative breast cancer cells[J]. Front Oncol. https://doi.org/10.3389/fonc.2022.819714
Wang J, Su N, Fang Y et al (2022) Comparison of chemotherapy combined with Chidamide versus chemotherapy in the frontline treatment for peripheral T-cell lymphoma[J]. Front Immunol 2(13):835103
Yin L, Zhang Q, Xie S et al (2023) HDAC inhibitor Chidamide overcomes drug resistance in chronic myeloid leukemia with the T315i mutation through the Akt-autophagy pathway[J]. Hum Cell 36(4):1564–1577
Chai Y, Chen B, Qi F et al (2022) First-line chemoradiation with or without Chidamide (tucidinostat) in patients with intermediate- and high-risk early-stage extranodal nasal-type natural killer/T-cell lymphoma: a randomized phase 2 study in China[J]. Int J Radiat Oncol Biol Phys 113(4):833–844
Wang F, Jin Y, Wang M et al (2024) Combined anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal cancer: a randomized phase 2 trial[J]. Nat Med 30(4):1035–1043
Funding
Funding for this study was received from the Graduate Education Innovation Project at Sun Yat-sen University in 2024, 02300-12240001, the Natural Science Foundation of Henan Province, 242301420078, the National Natural Science Foundation of China, Nos. U20A20369, the Shenzhen Science and Technology Program, KQTD20190929173853397, the "Pearl River Talent Plan" Innovation and Entrepreneurship Team Project of Guangdong Province, 2019ZT08Y464, and the Guangdong Basic and Applied Basic Research Foundation, 2022B1515120085.
Author information
Authors and Affiliations
Contributions
Y.G. conceived and designed the experiments. X.N., W.Z., and X.Z. performed the experiments. F.L., Y.X., Q.D., X.S., T.L, W.L., X.Y., Z.H., W.S., and Y.S. helped to perform (title page with author details) the experiments. X.N., W.Z., X.Z., and Y.G. analyzed and interpreted the results. All authors revised the manuscript, discussed the results, and gave final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Niu, X., Zhao, W., Zhou, X. et al. Chidamide functions as a VISTA/PSGL-1 blocker for cancer immunotherapy. Cancer Immunol Immunother 74, 104 (2025). https://doi.org/10.1007/s00262-025-03955-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00262-025-03955-y