Abstract
Background
We report dose-escalation results from an open-label, phase 1/2 trial evaluating avelumab (anti-PD-L1) in paediatric patients with refractory/relapsed solid tumours.
Methods
In phase 1, patients aged < 18 years with solid (including central nervous system [CNS]) tumours for which standard therapy did not exist or had failed were enrolled in sequential cohorts of 3–6 patients. Patients received avelumab 10 or 20 mg/kg intravenously every 2 weeks. Primary endpoints were dose-limiting toxicities (DLTs) and grade ≥ 3 treatment-emergent adverse events (AEs).
Results
At data cut-off (27 July 2021), 21 patients aged 3–17 years had received avelumab 10 mg/kg (n = 6) or 20 mg/kg (n = 15). One patient had three events that were classified as a DLT (fatigue with hemiparesis and muscular weakness associated with pseudoprogression; 20 mg/kg cohort). Grade ≥ 3 AEs occurred in five (83%) and 11 (73%) patients in the 10 and 20 mg/kg cohorts, respectively, and were treatment-related in one patient (7%; grade 3 [DLT]) in the 20 mg/kg cohort. Avelumab exposure in paediatric patients receiving 20 mg/kg dosing, but not 10 mg/kg, was comparable or higher compared with approved adult dosing (10 mg/kg or 800 mg flat dose). No objective responses were observed. Four patients with CNS tumours (20 mg/kg cohort) achieved stable disease, which was ongoing in two patients with astrocytoma at cut-off (for 24.7 and 30.3 months).
Conclusion
In paediatric patients with refractory/relapsed solid tumours, avelumab monotherapy showed a safety profile consistent with previous adult studies, but clinical benefits were limited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Treatment of advanced paediatric cancer typically includes cytotoxic chemotherapy; however, patients often develop resistance and have refractory or relapsed disease, resulting in a poor prognosis [1, 2]. Immune checkpoint inhibitors (ICIs) that target the PD-L1/PD-1 interaction have been approved as treatments for various adult cancers. Recently, several early phase trials investigating ICI monotherapy specifically in paediatric cancers have shown acceptable safety profiles but low antitumour activity, except in Hodgkin lymphoma [3,4,5].
Avelumab, an anti-PD-L1 antibody, has shown clinical activity in various tumours [6,7,8,9,10]. Avelumab is approved in various countries for the treatment of metastatic Merkel cell carcinoma (including patients aged ≥ 12 years in the USA) in addition to platinum-treated urothelial carcinoma (first-line maintenance therapy or second-line therapy) and advanced renal cell carcinoma (first-line treatment in combination with axitinib) [11]. Avelumab was initially approved with 10-mg/kg dosing every 2 weeks (Q2W), but this was subsequently changed to a flat dose of 800 mg in the USA, Europe, and other locations, based on pharmacokinetic (PK) studies [12]. Other ICIs approved specifically for paediatric patients are pembrolizumab (in primary mediastinal large B-cell lymphoma, microsatellite instability—high cancers, tumour mutational burden—high cancers, and Merkel cell carcinoma in the USA, and relapsed/refractory classical Hodgkin lymphoma in the USA and Europe) and nivolumab (alone or combined with ipilimumab in microsatellite instability—high metastatic colorectal cancer in the USA) [13, 14]. Except for Hodgkin lymphoma, ICI approvals in paediatric populations have generally been based on paediatric safety/PK analyses and efficacy findings in adults [13, 14].
We report dose-escalation results from a trial of avelumab monotherapy in paediatric patients with refractory or relapsed solid tumours.
Methods
Study design and participants
In phase 1 of this international, open-label, multicentre, single-arm, phase 1/2 trial (registered at clinicaltrials.gov: NCT03451825), eligible patients were aged < 18 years at first dose and had a histologically or cytologically confirmed diagnosis of a solid tumour (including central nervous system [CNS] tumours) or lymphoma that had progressed with standard therapy or for which no standard therapy existed. Other eligibility criteria included Lansky (≤ 16 years) or Karnofsky (> 16 years) performance status ≥ 50; estimated life expectancy > 3 months; adequate haematologic, hepatic, and renal function; availability of recently obtained tumour tissue; negative pregnancy test (in all postmenarcheal females, females aged ≥ 10 years, or per local guidelines); and use of effective contraception (in patients who were considered to be biologically capable of having children and were sexually active). Exclusion criteria included rapidly progressive disease (PD), grade ≥ 3 neuropathy, known congenital immunodeficiency, prior therapy targeting a T-cell coregulatory protein, active autoimmune disease that might deteriorate when receiving an immunostimulatory agent (not including diabetes type 1, vitiligo, psoriasis, or hypothyroid/hyperthyroid disease not requiring immunosuppressive treatment), and serious cardiovascular disease or other severe medical conditions. Use of systemic steroids was tapered before study treatment except for adrenal insufficiency (physiological replacement dose permitted) or acute allergy (≤ 14 days permitted).
The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki and the International Council for Harmonisation Guideline for Good Clinical Practice. The protocol was approved by the institutional review board or independent ethics committee of each centre. All patients or legal representatives of patients provided written informed consent before enrolment.
Procedures
Sequential cohorts of three to six patients were enrolled. The avelumab starting dose was 10 mg/kg by 1-h intravenous infusion Q2W. Escalation to, but not exceeding, 20 mg/kg intravenously Q2W was planned if exposure was inadequate compared with adult exposures derived from population PK simulations (maximum serum concentration, area under the concentration–time curve [AUC], and trough serum concentration [Ctrough]). To mitigate the potential for infusion-related reactions (IRRs), a known adverse event (AE) with avelumab [15], antihistamine (e.g. diphenhydramine) and paracetamol premedication, dosed per local treatment standards, was mandatory 30 to 60 min before the first four infusions.
AEs were coded using Medical Dictionary for Regulatory Activities (MedDRA) version 22.1 and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Dose-limiting toxicity (DLT) was defined as any of the following events occurring during the DLT observation period (first two cycles of treatment [28 days]) if considered related to avelumab: grade 4 neutropenia (> 7 days), thrombocytopenia (> 7 days), or anaemia; grade ≥ 3 neutropenic infection or thrombocytopenia with bleeding; or specified grade ≥ 3 nonhaematologic toxicities excluding those that resolved and/or were without clinical correlate. Inability to complete two or more avelumab infusions during the DLT period due to treatment-related toxicity was also classified as a DLT. All safety data were reviewed by the Safety Monitoring Committee (SMC) for potential DLTs at predefined intervals. The maximum tolerated dose (MTD) was defined as the highest dose level at which < 33% of evaluable patients experienced a DLT, provided that a higher dose level was tested and had an associated DLT rate ≥ 33%. Immune-related AEs were evaluated using a customised list of MedDRA terms and by investigator assessment using a predefined medical algorithm. IRRs were identified using prespecified lists of MedDRA terms in association with time of onset and resolution.
Patients received avelumab until confirmed PD per immune-related Response Evaluation Criteria in Solid Tumours (irRECIST), death, unacceptable toxicity, or withdrawal of consent. Treatment could continue after confirmed PD if the patient had no new or worsening symptoms, was tolerating avelumab, had stable performance status, and treatment would not delay preventive intervention for serious complications of PD. Tumours were assessed radiologically at baseline, every 8 weeks for 24 weeks, then every 12 weeks thereafter. Objective tumour response was evaluated by investigators per RECIST version 1.1. For some analyses, patients were assigned to subgroups of CNS tumours, sarcomas, and gastrointestinal (GI) tumours.
Blood samples for PK analysis were collected during treatment cycles 1, 2, 3, 5, 7, 8, 13, and every 6 cycles thereafter. Serum avelumab concentrations were analysed by immunoassay. PK parameters were calculated by noncompartmental analysis.
PD-L1 expression was assessed in baseline tumour tissue using the PD-L1 73–10 immunohistochemistry assay (Dako, Carpinteria, California, USA). PD-L1+ status was defined as PD-L1 expression on tumour cells at any intensity with cut-offs of ≥ 1%, ≥ 5%, ≥ 25%, ≥ 50%, or ≥ 80%.
Outcomes
Primary endpoints in phase 1 were DLTs in the DLT observation period, to determine the recommended phase 2 dose, and grade ≥ 3 treatment-emergent AEs. Secondary endpoints included confirmed best overall response and progression-free survival (PFS) per RECIST 1.1 by investigator assessment; overall survival (OS); safety; and single/multiple-dose PK profiles.
Statistical analysis
Efficacy and safety were analysed in all patients who received at least one avelumab dose. DLTs were evaluated in all patients who received all assigned trial treatment administrations in the DLT evaluation period or who stopped treatment because of DLTs in this period. Planned enrolment was 12 to 36 patients in phase 1 using the modified toxicity probability interval approach [16]. At least 12 DLT-evaluable patients, treated at a dose level confirmed to be safe, were required for the primary analysis. Two-sided 95% CIs for objective response rates were calculated using the Clopper–Pearson method. Time-to-event endpoints were estimated using the Kaplan–Meier method, and corresponding two-sided CIs for medians were calculated using the Brookmeyer–Crowley method.
Results
Patients
Twenty-one patients with various advanced solid tumours were enrolled. Most patients (71%) were Asian. Median age was 12.0 years (range 3–17), and median weight was 37.3 kg (range 13.4–78.7). Patients received avelumab 10 mg/kg (n = 6) or 20 mg/kg (n = 15) Q2W (Table 1). Tumour subgroups were CNS in eight (all 20 mg/kg cohort), sarcoma in 12 (10 mg/kg [n = 5] and 20 mg/kg [n = 7] cohorts), and GI in one (colon cancer; 10 mg/kg cohort). No patients with lymphoma were enrolled. All patients had received prior therapy; nine patients (43%) had received four or more prior lines of therapy (Table 1).
Median duration of treatment was 8.2 weeks (range 6.1–15.9) in the 10 mg/kg cohort and 11.9 weeks (range 2.0–134.1) in the 20 mg/kg cohort, and median follow-up was 18.8 weeks (range 6.4–62.3) and 30.1 weeks (range 3.6–139.0), respectively. At data cut-off (27 July 2021), no patient remained on treatment (Fig. 1). The most common reason for discontinuation was PD (10 mg/kg, n = 5 [83%]; 20 mg/kg, n = 7 [47%]) (Fig. 1).
Safety
One patient in the 20 mg/kg cohort was not included in the DLT analysis because they received only one dose of avelumab owing to an AE. Of the remaining 20 patients (10 mg/kg, n = 6; 20 mg/kg, n = 14), 18 completed the DLT-evaluable period, whereas two patients stopped treatment after receiving two doses of avelumab due to PD, who, therefore, were nonevaluable for DLTs. One patient (8%) in the 20 mg/kg cohort with a high-grade glioma experienced three concurrent events (fatigue with hemiparesis and muscular weakness associated with pseudoprogression; all grade 3) that were assessed as a DLT by the SMC. The MTD was not reached. During the DLT evaluation period, treatment-emergent AEs of any grade or causality occurred in all six patients (100%) in the 10 mg/kg cohort and 11 of 12 patients (92%) in the 20 mg/kg cohort, including grade ≥ 3 AEs in one patient (17%) and two patients (17%), respectively.
In the full patient group, AEs of any grade occurred in all 21 patients, including grade ≥ 3 AEs in five patients (83%) in the 10 mg/kg cohort and 11 patients (73%) in the 20 mg/kg cohort (Table 2). The most common grade ≥ 3 AEs (≥ 30%) were abdominal pain (n = 2 [33%]) in the 10 mg/kg cohort and disease progression (n = 5 [33%]) in the 20 mg/kg cohort. In the 10 and 20 mg/kg cohorts, serious AEs of any grade occurred in four patients (67%) and 12 patients (80%), respectively. AEs led to discontinuation in patients in the 20 mg/kg cohort only (n = 8 [53%]), including disease progression (n = 5 [33%]), and thrombocytopenia (n = 1 [7%]), malignant pleural effusion (n = 1 [7%]), and intracranial pressure increased (n = 1 [7%]), all three of which were related to disease progression. AEs resulted in death in one patient (17%) in the 10 mg/kg cohort and three patients (20%) in the 20 mg/kg cohort, all due to disease progression. Treatment-related AEs (TRAEs) of any grade occurred in three patients (50%) in the 10 mg/kg cohort and 10 patients (67%) in the 20 mg/kg cohort (Supplementary Table 1). The most common TRAEs (≥ 20%) in the 20 mg/kg cohort were fatigue (n = 4 [27%]), nausea (n = 3 [20%]), and chills (n = 3 [20%]); no TRAE occurred in more than one patient in the 10 mg/kg cohort. Grade 3 TRAEs occurred in one patient (7%) in the 20 mg/kg cohort (fatigue, hemiparesis, muscle weakness, and tumour pseudoprogression; patient with DLT described above). No grade 4 or 5 TRAEs occurred, and none led to discontinuation. An immune-related AE occurred in one patient (7%) in the 20 mg/kg cohort (grade 2 hypothyroidism). Grade 1/2 IRRs occurred in two patients (33%) in the 10 mg/kg cohort and seven patients (47%) in the 20 mg/kg cohort; no grade ≥ 3 IRRs occurred.
PK
In PK analyses (N = 21; data cut-off, 21 October 2019), the median and geometric mean of the AUC and Ctrough for cycle 1 in the 10 mg/kg cohort appeared lower vs approved adult dosing, particularly in patients with a body weight of < 40 kg (Table 3). The median and geometric mean of the AUC and Ctrough for cycle 1 in the 20 mg/kg cohort were similar or higher vs adult values with approved dosing, irrespective of body weight. No clear association was observed between age and exposure in either dose cohort. Additionally, the PK profile in the patient with DLT was similar to other patients in the same dose cohort (20 mg/kg) and adults treated with approved dosing.
Efficacy
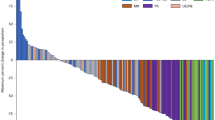
No objective responses were observed (Supplementary Tables 2 and 3). No patient had a reduction in the sum of target lesions (Fig. 2; Supplementary Fig. 1), and no trend in type of progression (i.e. target vs nontarget vs new lesion) was observed (Supplementary Table 4). Four patients in the 20 mg/kg cohort had stable disease (SD). The disease control rate (proportion with confirmed response or SD) was 0% (95% CI, 0–46) in the 10 mg/kg cohort and 27% (95% CI, 8–55) in the 20 mg/kg cohort. All four patients who had SD had a CNS tumour: astrocytoma of the spinal cord, pilocytic astrocytoma, pilomyxoid astrocytoma (all low grade), and H3 K27M-mutant diffuse midline glioma. Duration of SD ranged from 2.4 to 30.3 months and was ongoing at last assessment (data cut-off, 27 July 2021) in two patients with astrocytoma after 30.3 and 24.7 months (Supplementary Fig. 2A). Prestudy target lesion data suggested that these tumours were growing slowly prior to study entry (Supplementary Fig. 2B). Prior systemic treatment or radiotherapy and site of primary tumour were not associated with clinical benefit from avelumab (Supplementary Table 5).
Change in target lesions per RECIST 1.1 from baseline over time in evaluable patients (those with baseline and postbaseline data): A all evaluable patients (n = 18); B patients with central nervous system tumours (n = 7). Increases greater than 200% are shown as 200%. PD, progressive disease; RECIST 1.1, Response Evaluation Criteria in Solid Tumours version 1.1
Median PFS was 7.5 weeks (95% CI, 6.6–not estimable [NE]) in the 10 mg/kg cohort and 7.7 weeks (95% CI, 2.3–10.3) in the 20 mg/kg cohort (Supplementary Fig. 3A); median OS was 4.4 months (95% CI, 1.5–NE) and 7.0 months (95% CI, 1.6–10.8), respectively (Supplementary Fig. 3B).
Biomarker analyses
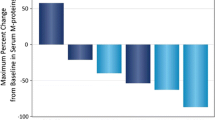
A total of 15 patients were evaluable for PD-L1 expression (Supplementary Table 6). Using a ≥ 1% cut-off to define PD-L1+ status, five patients (33%) had PD-L1+ tumours and 10 (67%) had PD-L1− tumours. Notably, the two patients with astrocytoma who had prolonged and ongoing SD with avelumab treatment had tumours with high PD-L1+ expression at baseline (≥ 80% of tumour cells; Fig. 3). The other three patients with PD-L1+ tumours all had PD as their best overall response with avelumab.
Histological images of H&E and PD-L1 (73–10) staining of tumour samples for the two patients with astrocytoma who had prolonged SD (> 24 months) with avelumab. Both patients had tumours with ≥ 80% of tumour cells having membrane staining positive for PD-L1 expression. Patient A (female aged 9 years) had a pilocytic astrocytoma (WHO grade I). The patient presented in 2018 with a mass at the cerebellopontine angle and upper cervical spine showing cystic and contrast-enhancing solid portions via MRI scan. Histopathology showed an astrocytic tumour with increased cellularity, mild pleomorphism, low mitotic activity (2/10 high-power fields), and absent necrosis. Immunophenotype was positive for glial fibrillary acidic protein and strong PD-L1 expression in tumour cells but not tumour vessels (arrow). The tumour was BRAFV600E mutation-positive, but no PTEN deletion or MGMT promoter methylation was present. The patient underwent surgery in April 2018 with residual tumour and received vincristine + carboplatin from May to July 2018 (best overall response of PD) followed by thioguanine + procarbazine + lomustine in August 2018 (best overall response unknown); no radiation was administered. The patient received avelumab treatment from October 2018 until April 2021, and tumour size changed over time from 40 to 45 mm. Lansky performance status improved from 50% at study entry to 70% with avelumab treatment, and the patient discontinued from the study to receive subsequent anticancer therapy (surgery). Patient B (male aged 3 years) had an astrocytoma of spinal cord (WHO grade II; NF1-associated). The patient presented in 2018 with a contrast-enhancing intramedullary mass at the upper thoracic spinal cord (MRI). Microscopy showed a tumour with increased cellularity, mild pleomorphism, low mitotic activity (1/10 high-power field), absent microvascular proliferation, and absent necrosis. Immunophenotype was positive for glial fibrillary acidic protein and synaptophysin, and strong PD-L1 expression was seen in tumour cells but not tumour vessels (arrow). The tumour had an NF1 mutation (p.Gln1577*, c.4729C > T), but no mutations of BRAF (V600E), IDH1, TP53, or PTEN were present. In 2021, the tumour was classified as ganglioglioma, WHO grade I. The patient had surgery with residual tumour in July 2018 and received vincristine + carboplatin from August to November 2018 (best overall response of PD), with no radiation. The patient received avelumab treatment from December 2018 until February 2021, and tumour size changed over time from 12 to 16 mm (not classified as PD according to RECIST 1.1 because the tumour size did not increase by ≥ 5 mm), with Lansky performance status stable at 90%. The patient discontinued the study to receive subsequent anticancer therapy (surgery). H&E, haematoxylin and eosin; MRI, magnetic resonance imaging; PD, progressive disease; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease; and WHO, World Health Organization
Discussion
Avelumab monotherapy had an acceptable safety profile in paediatric patients at both dose levels investigated, 10 mg/kg and 20 mg/kg Q2W, with a low incidence of grade ≥ 3 TRAEs and no grade ≥ 3 immune-related AEs. One patient treated with 20 mg/kg had three concurrent events that were assessed as a DLT but were likely associated with tumour pseudoprogression, a known phenomenon with ICI treatment [17] that may not be dose dependent. The MTD was not reached, which has been a common finding in ICI trials and reflects the challenges of dose evaluation using study designs adopted initially for cytotoxic agents. Grade ≥ 3 AEs occurred in 83% in the 10 mg/kg cohort and 73% in the 20 mg/kg cohort, but grade ≥ 3 AEs were considered treatment-related only in one patient (DLT; 20 mg/kg cohort), and no grade 4/5 TRAEs occurred. All AEs that led to discontinuation were associated with disease progression. Grade 1/2 IRRs occurred in 33% in the 10 mg/kg cohort and 47% in the 20 mg/kg cohort, and no grade ≥ 3 IRRs occurred. These rates appear higher than those seen in studies of avelumab in adults, although this may be due to the small sample size in the paediatric study [15]. The frequency of IRRs in this study was also higher than reported for other ICIs in trials in paediatric patients, although it should be noted that trials of other ICIs used narrower definitions for IRR [3, 4]. No new safety signals were observed in paediatric patients, and the frequency and severity of AEs were generally consistent with adult studies [15].
PK analysis showed that paediatric dosing with 10 mg/kg resulted in lower exposure vs adults receiving approved dosing (10 mg/kg or 800 mg Q2W), particularly in patients weighing < 40 kg (i.e. those receiving the lowest dose). However, 20 mg/kg Q2W dosing achieved or exceeded exposures in adults, irrespective of body weight. PK analyses from this study, in addition to subsequent modelling and simulation approaches, have been used to select the recommended dose for future avelumab studies in paediatric patients of 15 mg/kg Q2W for patients < 12 years or < 40 kg and the adult dose of 800 mg Q2W for paediatric patients ≥ 12 years and ≥ 40 kg [18].
Antitumour activity of avelumab monotherapy was limited in relapsed or refractory paediatric solid tumours, consistent with other recent studies of ICIs in similar populations [3,4,5]. Four patients with CNS tumours achieved SD on study, including two patients with low-grade astrocytoma who had ongoing SD lasting > 24 months; however, prestudy tumour assessments suggested that these tumours were growing slowly. The lack of objective responses reported with several ICIs may be due to differences in tumour biology between paediatric and adult cancers, including a lower mutational burden in most paediatric tumours [19], and differences in immune responses between adults and younger patients [20]. Additionally, the enrolled population had a high proportion of patients who were Asian, which may have introduced bias. Despite recruitment efforts, no patients were enrolled with lymphoma, a malignancy that often responds to ICI monotherapy [3,4,5]. Limited data are available on paediatric patients with CNS tumours treated with other ICIs because trials generally exclude these patients [4, 5]. In KEYNOTE-051, pembrolizumab showed some benefit in paediatric patients with various PD-L1+ solid tumours, including partial response in a patient with a malignant ganglioglioma, and tumour shrinkage (< 30% decrease) in patients with high-grade glioma, glioblastoma, ependymoma, and ganglioneuroblastoma among other tumours [3]. In this study, two of the three patients with astrocytoma who had prolonged SD with avelumab had high PD-L1+ tumours (≥ 80%); the other three patients with PD-L1+ tumours had PD as best response to avelumab.
This study was part of a paediatric investigation plan approved by the European Medicines Agency in 2017, which was originally for the treatment of solid tumours and was subsequently modified to include lymphomas and CNS tumours [21]. The trial was initiated, and planned as a phase 1/2 study, before the updated overall paediatric strategy for ICIs was agreed upon by ACCELERATE and the European Medicines Agency at the Paediatric Strategy Forum in September 2018, which recommended a focus on combination studies because of the limited activity seen in several studies with ICI monotherapy [22]. Subsequently, it was decided not to proceed with phase 2 after the completion of phase 1 of this trial. A future study will investigate the combination of avelumab plus lenvatinib (a receptor tyrosine kinase inhibitor) in paediatric patients with CNS tumours (NCT05081180). This planned trial is supported by the disease stabilizations observed both in our trial and in a retrospective study of ICIs [23]. Additionally, in a cohort of 31 adults with previously treated glioblastoma multiforme who received lenvatinib in combination with pembrolizumab within a phase 2 trial, an objective response rate of 16% (per Response Assessment in Neuro-Oncology criteria) and disease control rate of 58% were reported [24], supporting the evaluation of lenvatinib and avelumab combination therapy in paediatric patients with CNS tumours.
In conclusion, the tolerability seen with avelumab monotherapy in paediatric patients with previously treated solid tumours, including those with CNS tumours, supports further studies of avelumab-based combination therapy in these tumours.
Availability of data and material
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck’s Data Sharing Policy. All requests should be submitted in writing to Merck’s data sharing portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavour to gain agreement to share data in response to requests.
Abbreviations
- AE:
-
Adverse event
- AUC:
-
Area under the concentration–time curve
- CNS:
-
Central nervous system
- Ctrough :
-
Trough serum concentration
- DLT:
-
Dose-limiting toxicity
- GI:
-
Gastrointestinal
- H&E:
-
Haematoxylin and eosin
- ICI:
-
Immune checkpoint inhibitor
- IRR:
-
Infusion-related reaction
- irRECIST:
-
Immune-related Response Evaluation Criteria in Solid Tumours
- MedDRA:
-
Medical Dictionary for Regulatory Activities
- MRI:
-
Magnetic resonance imaging
- MTD:
-
Maximum tolerated dose
- NE:
-
Not estimable
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PK:
-
Pharmacokinetic
- Q2W:
-
Every 2 weeks
- RECIST:
-
Response Evaluation Criteria in Solid Tumours
- SD:
-
Stable disease
- SMC:
-
Safety Monitoring Committee
- TRAE:
-
Treatment-related adverse event
- WHO:
-
World Health Organization
References
Cho HW, Lee JW, Ma Y, Yoo KH, Sung KW, Koo HH (2018) Treatment outcomes in children and adolescents with relapsed or progressed solid tumors: a 20-year, single-center study. J Korean Med Sci 33(41):e260. https://doi.org/10.3346/jkms.2018.33.e260
Majzner RG, Heitzeneder S, Mackall CL (2017) Harnessing the immunotherapy revolution for the treatment of childhood cancers. Cancer Cell 31(4):476–485. https://doi.org/10.1016/j.ccell.2017.03.002
Geoerger B, Kang HJ, Yalon-Oren M, Marshall LV, Vezina C, Pappo A, Laetsch TW, Petrilli AS, Ebinger M, Toporski J, Glade-Bender J, Nicholls W, Fox E, DuBois SG, Macy ME, Cohn SL, Pathiraja K, Diede SJ, Ebbinghaus S, Pinto N (2020) Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol 21(1):121–133. https://doi.org/10.1016/S1470-2045(19)30671-0
Geoerger B, Zwaan CM, Marshall LV, Michon J, Bourdeaut F, Casanova M, Corradini N, Rossato G, Farid-Kapadia M, Shemesh CS, Hutchinson KE, Donaldson F, Liao M, Caron H, Trippett T (2020) Atezolizumab for children and young adults with previously treated solid tumours, non-Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): a multicentre phase 1–2 study. Lancet Oncol 21(1):134–144. https://doi.org/10.1016/S1470-2045(19)30693-X
Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, Minard CG, Voss S, Berg SL, Weigel BJ, Mackall CL (2020) Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol 21(4):541–550. https://doi.org/10.1016/S1470-2045(20)30023-1
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, Lee JL, Vasiliev A, Miller WH Jr, Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK (2019) Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1103–1115. https://doi.org/10.1056/NEJMoa1816047
Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbe C, Linette GP, Milella M, Brownell I, Lewis KD, Lorch JH, Chin K, Mahnke L, von Heydebreck A, Cuillerot JM, Nghiem P (2016) Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 17(10):1374–1385. https://doi.org/10.1016/S1470-2045(16)30364-3
Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulovic S, Demey W, Ullen A, Loriot Y, Sridhar SS, Tsuchiya N, Kopyltsov E, Sternberg CN, Bellmunt J, Aragon-Ching JB, Petrylak DP, Laliberte R, Wang J, Huang B, Davis C, Fowst C, Costa N, Blake-Haskins JA, di Pietro A, Grivas P (2020) Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383(13):1218–1230. https://doi.org/10.1056/NEJMoa2002788
Keilholz U, Mehnert JM, Bauer S, Bourgeois H, Patel MR, Gravenor D, Nemunaitis JJ, Taylor MH, Wyrwicz L, Lee KW, Kasturi V, Chin K, von Heydebreck A, Gulley JL (2019) Avelumab in patients with previously treated metastatic melanoma: phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer 7(1):12. https://doi.org/10.1186/s40425-018-0459-y
Hassan R, Thomas A, Nemunaitis JJ, Patel MR, Bennouna J, Chen FL, Delord JP, Dowlati A, Kochuparambil ST, Taylor MH, Powderly JD, Vaishampayan UN, Verschraegen C, Grote HJ, von Heydebreck A, Chin K, Gulley JL (2019) Efficacy and safety of avelumab treatment in patients with advanced unresectable mesothelioma: phase 1b results from the JAVELIN Solid Tumor trial. JAMA Oncol 5(3):351–357. https://doi.org/10.1001/jamaoncol.2018.5428
Bavencio (avelumab) Prescribing information. EMD Serono; (2020)
Novakovic AM, Wilkins JJ, Dai H, Wade JR, Neuteboom B, Brar S, Bello CL, Girard P, Khandelwal A (2020) Changing body weight-based dosing to a flat dose for avelumab in metastatic Merkel cell and advanced urothelial carcinoma. Clin Pharmacol Ther 107(3):588–596. https://doi.org/10.1002/cpt.1645
Keytruda (pembrolizumab) Prescribing information. Whitehouse Station, Merck & Co., Inc, NJ (2022)
Opdivo (nivolumab) Prescribing information. Princeton, NJ; Bristol-Myers Squibb (2021)
Kelly K, Infante JR, Taylor MH, Patel MR, Wong DJ, Iannotti N, Mehnert JM, Loos AH, Koch H, Speit I, Gulley JL (2018) Safety profile of avelumab in patients with advanced solid tumors: a pooled analysis of data from the phase 1 JAVELIN Solid Tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer 124(9):2010–2017. https://doi.org/10.1002/cncr.31293
Ji Y, Wang SJ (2013) Modified toxicity probability interval design: a safer and more reliable method than the 3 + 3 design for practical phase I trials. J Clin Oncol 31(14):1785–1791. https://doi.org/10.1200/JCO.2012.45.7903
Wang Q, Gao J, Wu X (2018) Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol 58:125–135. https://doi.org/10.1016/j.intimp.2018.03.018
Vugmeyster Y, Grisic A-M, Brockhaus B, Rueckert P, Ruisi M, Dai H, Khandelwal A (in press) Avelumab dose selection for clinical studies in pediatric patients with solid tumors. Clin Pharmacokinet
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortes ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CWM, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G (2013) Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499(7457):214–218. https://doi.org/10.1038/nature12213
Rauwolf KK, Rossig C (2019) Redirecting T cells to treat solid pediatric cancers. Cancer Metastasis Rev 38(4):611–624. https://doi.org/10.1007/s10555-019-09821-5
European Medicines Agency decision P/0361/2017 of 1 December 2017. https://www.ema.europa.eu/en/documents/pip-decision/p/0361/2017-ema-decision-1-december-2017-acceptance-modification-agreed-paediatric-investigation-plan_en.pdf. Accessed March 4 2021
Pearson ADJ, Rossig C, Lesa G, Diede SJ, Weiner S, Anderson J, Gray J, Geoerger B, Minard-Colin V, Marshall LV, Smith M, Sondel P, Bajars M, Baldazzi C, Barry E, Blackman S, Blanc P, Capdeville R, Caron H, Cole PD, Jimenez JC, Demolis P, Donoghue M, Elgadi M, Gajewski T, Galluzzo S, Ilaria R Jr, Jenkner A, Karres D, Kieran M, Ligas F, Lowy I, Meyers M, Oprea C, Peddareddigari VGR, Sterba J, Stockman PK, Suenaert P, Tabori U, van Tilburg C, Yancey T, Weigel B, Norga K, Reaman G, Vassal G (2020) ACCELERATE and European Medicines Agency Paediatric Strategy Forum for medicinal product development of checkpoint inhibitors for use in combination therapy in paediatric patients. Eur J Cancer 127:52–66. https://doi.org/10.1016/j.ejca.2019.12.029
Cacciotti C, Choi J, Alexandrescu S, Zimmerman MA, Cooney TM, Chordas C, Clymer J, Chi S, Yeo KK (2020) Immune checkpoint inhibition for pediatric patients with recurrent/refractory CNS tumors: a single institution experience. J Neurooncol 149(1):113–122. https://doi.org/10.1007/s11060-020-03578-6
Lwin Z, Gomez-Roca C, Saada-Bouzid E, Yanez E, Muñoz FL, Im SA, Castanon E, Senellart H, Graham D, Voss M, Doherty M, Lopez J, Ghori R, Kubiak P, Jin F, Norwood K, Chung HC (2020) LBA41 LEAP-005: Phase II study of lenvatinib (len) plus pembrolizumab (pembro) in patients (pts) with previously treated advanced solid tumours. Ann Oncol. https://doi.org/10.1016/j.annonc.2020.08.2271
Acknowledgements
The authors thank the patients and their families, investigators, coinvestigators, and the study teams at each of the participating centres. The authors would also like to thank Karin Tyroller for supporting the data analyses, and Sara Georges and Hans Jürgen Grote for PD-L1 immunohistochemistry analyses and collating information and images for the associated case reports. Medical writing support was provided by Amy Davidson of ClinicalThinking and was funded by Merck (CrossRef Funder ID: 10.13039/100009945) and Pfizer.
Funding
This study was sponsored by Merck (CrossRef Funder ID: 10. 13039/100009945), as part of an alliance between Merck and Pfizer. Medical writing support was funded by Merck and Pfizer.
Author information
Authors and Affiliations
Contributions
All authors contributed to data collection, investigation, and manuscript development. DML, JX, PP, IJ, YV, MR, and HJK contributed to study design. JX, PP, IJ, YV, and MR contributed to data curation, data analysis, and methodology.
Corresponding author
Ethics declarations
Conflict of interest
DAM reports serving as a consultant or advisor for Bayer, Boehringer Ingelheim, Clarity Pharmaceuticals, Roche, and Y-mAbs Therapeutics; is a member of a speakers bureau for EUSA Pharma; has received institutional research funding from Bristol Myers Squibb; and has received reimbursement for travel and accommodation expenses from EUSA Pharma and Y-mAbs Therapeutics. KN has received honoraria from and reports serving as a consultant or advisor for Bayer and Y-mAbs Therapeutics. MEM reports serving as a consultant or advisor for Ventana Medical Systems; has received institutional research funding from Bayer, Ignyta, Lilly, MSD, and Roche; holds stock in GE Healthcare, Johnson & Johnson, Teva Pharmaceuticals, and Varian Medical Systems; and has a patent for non-invasive methods of leukaemia cell detection with magnetic resonance imaging/magnetic resonance spectroscopy (US patent 8,894,975). JX was employed by EMD Serono Research & Development Institute Inc., Billerica, MA, USA, an affiliate of Merck KGaA at the time of manuscript preparation. PP and IJ report employment with Merck Healthcare KGaA, Darmstadt, Germany. YV reports employment with EMD Serono Research & Development Institute Inc., Billerica, MA, USA, an affiliate of Merck KGaA. MR reports employment with EMD Serono Research & Development Institute Inc., Billerica, MA, USA, an affiliate of Merck KGaA at the time of manuscript preparation, and holds stock in Bristol Myers Squibb. All other authors declare no competing interests.
Ethical approval
The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki and the International Council for Harmonisation Guideline for Good Clinical Practice. The protocol was approved by the institutional review board or independent ethics committee of each centre. All patients or legal representatives of patients provided written informed consent before enrolment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loeb, D.M., Lee, J.W., Morgenstern, D.A. et al. Avelumab in paediatric patients with refractory or relapsed solid tumours: dose-escalation results from an open-label, single-arm, phase 1/2 trial. Cancer Immunol Immunother 71, 2485–2495 (2022). https://doi.org/10.1007/s00262-022-03159-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03159-8