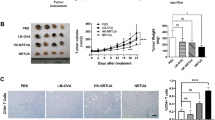
Abstract
Echinococcus granulosus is a cestode parasite which causes cystic echinococcosis disease. Previously we observed that vaccination with E. granulosus antigens from human hydatid cyst fluid (HCF) significantly inhibits colon cancer growth. In the present work, we evaluate the anti-tumor immune response induced by human HCF against LL/2 lung cancer in mice. HCF vaccination protected from tumor growth, both in prophylactic and therapeutic settings, and significantly increased mouse survival compared to control mice. Considering that tumor-associated carbohydrate antigens are expressed in E. granulosus, we oxidized terminal carbohydrates in HCF with sodium periodate. This treatment abrogates the anti-tumor activity induced by HCF vaccination. We found that HCF vaccination-induced IgG antibodies that recognize LL/2 tumor cells by flow cytometry. An antigen-specific immune response is induced with HCF vaccination in the tumor-draining lymph nodes and spleen characterized by the production of IL-5 and, in less extent, IFNɣ. In the tumor microenvironment, we found that NK1.1 positive cells from HCF-treated mice showed higher expression of CD69 than control mice ones, indicating a higher level of activation. When we depleted these cells by administrating the NK-specific antibody NK1.1, a significantly decreased survival was observed in HCF-induced mice, suggesting that NK1.1+ cells mediate the anti-tumor protection induced by HCF. These results suggest that HCF can evoke an integrated anti-tumor immune response involving both, the innate and adaptive components, and provide novel insights into the understanding of the intricate relationship between HCF vaccination and tumor growth.






Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
Hirsch FR, Suda K, Wiens J, Bunn PA Jr (2016) New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 388:1012–1024. https://doi.org/10.1016/S0140-6736(16)31473-8
Du L, Herbst RS, Morgensztern D (2017) Immunotherapy in lung cancer. Hematol Oncol Clin North Am 31:131–141. https://doi.org/10.1016/j.hoc.2016.08.004
Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, Melero I, Schalper KA, Herbst RS (2019) Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-18-1538
Schlom J, Hodge JW, Palena C, Tsang KY, Jochems C, Greiner JW, Farsaci B, Madan RA, Heery CR, Gulley JL (2014) Therapeutic cancer vaccines. Adv Cancer Res 121:67–124. https://doi.org/10.1016/B978-0-12-800249-0.00002-0
Thomas A, Giaccone G (2015) Why has active immunotherapy not worked in lung cancer? Ann Oncol 26:2213–2220. https://doi.org/10.1093/annonc/mdv323
Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS (2015) Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol 35:S185–S198. https://doi.org/10.1016/j.semcancer.2015.03.004
Tuccitto A, Shahaj E, Vergani E, Ferro S, Huber V, Rodolfo M, Castelli C, Rivoltini L, Vallacchi V (2019) Immunosuppressive circuits in tumor microenvironment and their influence on cancer treatment efficacy. Virchows Arch 474:407–420. https://doi.org/10.1007/s00428-018-2477-z
Osinaga E (2007) Expression of cancer-associated simple mucin-type O-glycosylated antigens in parasites. Crit Rev Iubmb Life 59:269–273. https://doi.org/10.1080/15216540601188553
Darani HY, Yousefi M (2012) Parasites and cancers: parasite antigens as possible targets for cancer immunotherapy. Future Oncol 8:1529–1535. https://doi.org/10.2217/fon.12.155
Coley WB (1928) End results in Hodgkin’s disease and lymphosarcoma treated by the mixed toxins of Erysipelas and Bacillus prodigiosus, alone or combined with radiation. Ann Surg 88:641–667. https://doi.org/10.1097/00000658-192810000-00002
Chou R, Selph S, Buckley DI, Fu R, Griffin JC, Grusing S, Gore JL (2017) Intravesical therapy for the treatment of nonmuscle invasive bladder cancer: a systematic review and meta-analysis. J Urol 197:1189–1199. https://doi.org/10.1016/j.juro.2016.12.090
Ubillos L, Freire T, Berriel E, Chiribao ML, Chiale C, Festari MF, Medeiros A, Mazal D, Rondán M, Bollati-Fogolín M, Rabinovich GA, Robello C, Osinaga E (2016) Trypanosoma cruzi extracts elicit protective immune response against chemically induced colon and mammary cancers. Int J Cancer 138:1719–1731. https://doi.org/10.1002/ijc.29910
Baird JR, Byrne KT, Lizotte PH, Toraya-Brown S, Scarlett UK, Alexander MP, Sheen MR, Fox BA, Bzik DJ, Bosenberg M, Mullins DW, Turk MJ, Fiering S (2013) Immune-mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J Immunol 190:469–478. https://doi.org/10.4049/jimmunol.1201209
Chen L, He Z, Qin L, Li Q, Shi X, Zhao S, Chen L, Zhong N, Chen X (2011) Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity. PLoS ONE 6:e24407. https://doi.org/10.1371/journal.pone.0024407
Akgül H, Tez M, Unal AE, Keşkek M, Sayek I, Ozçelik T (2003) Echinococcus against cancer: why not? Cancer 98(9):1999–2000. https://doi.org/10.1002/cncr.11752
Berriel E, Russo S, Monin L, Festari MF, Berois N, Fernández G, Freire T, Osinaga E (2013) Anti-tumor activity of human hydatid cyst fluid in a murine model of colon cancer. Sci World J. https://doi.org/10.1155/2013/230176
Noya V, Bay S, Festari MF, García E, Rodriguez E, Chiale C, Ganneau C, Baleux F, Astrada S, Bollati-Fogolín M, Osinaga E, Freire T (2013) Mucin-like peptides from Echinococcus granulosus induce antitumor activity. Int J Oncol 43:775–784. https://doi.org/10.3892/ijo.2013.2000
Rodríguez E, Noya V, Cervi L, Chiribao ML, Brossard N, Chiale C, Carmona C, Giacomini C, Freire T (2015) Glycans from Fasciola hepatica modulate the host immune response and TLR-induced maturation of dendritic cells. PLoS Negl Trop Dis 9:e0004234. https://doi.org/10.1371/journal.pntd.0004234
Tawill S, le Goff L, Ali F, Blaxter M, Allen J (2004) Both free-living and parasitic nematodes induce a characteristic Th2 response that is dependent on the presence of intact glycans. Infect Immun 72:398–407. https://doi.org/10.1128/IAI.72.1.398-407.2004
Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G, Cummings RD, Kraal G, van Die I (2013) Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int J Parasitol 43:191–200. https://doi.org/10.1016/j.ijpara.2012.10.021
Karadayi S, Arslan S, Sumer Z, Turan M, Sumer H, Karadayi K (2013) Does hydatid disease have protective effects against lung cancer? Mol Biol Rep 40:4701–4704. https://doi.org/10.1007/s11033-013-2565-8
Alvarez Errico D, Medeiros A, Míguez M, Casaravilla C, Malgor R, Carmona C, Nieto A, Osinaga E (2001) O-glycosylation in Echinococcus granulosus: identification and characterization of the carcinoma associated Tn antigen. Exp Parasitol 98:100–109. https://doi.org/10.1006/expr.2001.4620
Apostolopoulos V, McKenzie IFC (2017) Cellular mucins: targets for Immunotherapy. Crit Rev Immunol 37:421–437. https://doi.org/10.1615/CritRevImmunol.v37.i2-6.110
Kim N, Lee HH, Lee HJ, Choi WS, Lee J, Kim HS (2019) Natural killer cells as a promising therapeutic target for cancer immunotherapy. Arch Pharm Res. https://doi.org/10.1007/s12272-019-01143-y
Soo RA, Chen Z, Yan Teng RS, Tan HL, Iacopetta B, Tai BC, Soong R (2018) Prognostic significance of immune cells in non-small cell lung cancer: meta-analysis. Oncotarget 9:24801–24820. https://doi.org/10.18632/oncotarget.24835
Niederkorn JY, Stewart GL, Ghazizadeh S, Mayhew E, Ross J, Fischer B (1988) Trichinella pseudospiralis larvae express natural killer (NK) cell-associated asialo-GM1 antigen and stimulate pulmonary NK activity. Infect Immun 56:1011–1016
Babu S, Blauvelt CP, Nutman TB (2007) Filarial parasites induce NK cell activation, type 1 and type 2 cytokine secretion, and subsequent apoptotic cell death. J Immunol 179:2445–2456. https://doi.org/10.4049/jimmunol.179.4.2445
Babu S, Porte P, Klei TR, Shultz LD, Rajan TV (1998) Host NK cells are required for the growth of the human filarial parasite Brugia malayi in mice. J Immunol 161:1428–1432
Hernández A, O’Connor JE, Mir A (1999) Phenotypic analysis of peripheral lymphocyte subpopulations in hydatid patients. Parasitol Res 85:948–950
Korten S, Volkmann L, Saeftel M, Fischer K, Taniguchi M, Fleischer B, Hoerauf A (2002) Expansion of NK cells with reduction of their inhibitory Ly-49A, Ly-49C, and Ly-49G2 receptor-expressing subsets in a murine helminth infection: contribution to parasite control. J Immunol 168:5199–5206. https://doi.org/10.4049/jimmunol.168.10.5199
Mourglia-Ettlin G, Marqués JM, Chabalgoity JA, Dematteis S (2011) Early peritoneal immune response during Echinococcus granulosus establishment displays a biphasic behavior. PLoS Negl Trop Dis 5:e1293. https://doi.org/10.1371/journal.pntd.0001293
Takatsu K (2011) Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci 87:463–485. https://doi.org/10.2183/pjab.87.463
Rosenberg HF, Dyer KD, Foster PS (2013) Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 13:9–22. https://doi.org/10.1038/nri3341
Capobianco A, Manfredi AA, Monno A, Rovere-Querini P, Rugarli C (2008) Melanoma and lymphoma rejection associated with eosinophil infiltration upon intratumoral injection of dendritic and NK/LAK cells. J Immunother 31:458–465. https://doi.org/10.1097/CJI.0b013e318174a512
Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hämmerling GJ (2015) Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol 16:609–617. https://doi.org/10.1038/ni.3159
Buonocore S, Haddou NO, Moore F, Florquin S, Paulart F, Heirman C, Thielemans K, Goldman M, Flamand V (2008) Neutrophil-dependent tumor rejection and priming of tumoricidal CD8+ T cell response induced by dendritic cells overexpressing CD95L. J Leukoc Biol 84:713–720. https://doi.org/10.1189/jlb.0108075
Garley M, Jabłońska E, Dąbrowska D (2016) NETs in cancer. Tumour Biol 37:14355–14361. https://doi.org/10.1007/s13277-016-5328-z
Riganò R, Profumo E, di Felice G, Ortona E, Teggi A, Siracusano A (1995) In vitro production of cytokines by peripheral blood mononuclear cells from hydatid patients. Clin Exp Immunol 99:433–439. https://doi.org/10.1111/j.1365-2249.1995.tb05569.x
Kanan JH, Chain BM (2006) Modulation of dendritic cell differentiation and cytokine secretion by the hydatid cyst fluid of Echinococcus granulosus. Immunology 118:271–278. https://doi.org/10.1111/j.1365-2567.2006.02375.x
Acknowledgements
We thank the Flow Cytometry Facility of Institut Pasteur de Montevideo for their assistance.
Funding
This work was supported by Fondo María Viñas, Agencia Nacional de Investigación e Innovación, Uruguay [Number FMV_1_2017_1_136442] (to EO), Fondo ANII-GSK, Agencia Nacional de Investigación e Innovación, Uruguay [Number FSGSK_1_2017_1_145466] (to EO), Programa Grupos de Investigación, CSIC, Universidad de la República, Uruguay [number 908] (to EO), Fondo para la Convergencia Estructural del MERCOSUR [COF 03/11] (to EB, GFG, MC, NB and EO).
Author information
Authors and Affiliations
Contributions
EO, TF, and EB designed the study. EB, TF, CC, ER, GFG, and NB performed experiments and analyzing the data. MC, GM and EO contributed to analysis and interpretation. TF, EO, and EB analyzed the data and wrote the manuscript. GM, NB and MC critically revised the manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All mice were maintained under specific pathogen-free conditions and used in accordance with protocols established by the Animal Care Committee of the Institut Pasteur de Montevideo, Uruguay. All work with mice was performed according to protocol # 010/12 approved by the Institutional Animal Care Committee and following the National Law of Animal Experimentation (# 18.611).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Berriel, E., Freire, T., Chiale, C. et al. Human hydatid cyst fluid-induced therapeutic anti-cancer immune responses via NK1.1+ cell activation in mice. Cancer Immunol Immunother 70, 3617–3627 (2021). https://doi.org/10.1007/s00262-021-02948-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02948-x