Abstract
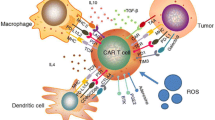
Chimeric antigen receptor (CAR) T cell therapy, a type of adoptive cell therapy, has been successfully used when treating lymphoma malignancies, but not nearly as successful in treating solid tumors. Trophoblast cell surface antigen 2 (Trop2) is expressed in various solid tumors and plays a role in tumor growth, invasion, and metastasis. In this study, a CAR targeting Trop2 (T2-CAR) was developed with different co-stimulatory intercellular domains. T2-CAR T cells demonstrated a powerful killing ability in the presence of Trop2-positive cells following an in vitro assay. Moreover, T2-CAR T cells produced multiple effector cytokines under antigen stimulation. In tumor-bearing mouse models, the CD27-based T2-CAR T cells showed a higher antitumor activity. Additionally, more CD27-based T2-CAR T cells survived in tumor-bearing mice spleens as well as in the tumor tissue. CD27-based T2-CAR T cells were also found to upregulate IL-7Rα expression, while downregulating PD-1 expression. In conclusion, the CD27 intercellular domain can enhance the T2-CAR T cell killing effect via multiple mechanisms, thus indicating that a CD27-based T2-CAR T cell approach is suitable for clinical applications.






Similar content being viewed by others
Availability of data and materials
All data generated during this study have included in this article.
Change history
08 May 2024
Missing supplementary file and the supplementary figure links which were wrongly linked to main text figures have been updated.
Abbreviations
- ACT:
-
Adoptive cell transfer
- Trop2:
-
Trophoblast cell surface antigen 2
- CAR:
-
Chimeric antigen receptor
- T2-CAR:
-
Trop2-specific CAR
- TAA:
-
Tumor-associated antigen
- FDA:
-
Food and Drug Administration
- scFv:
-
Single-chain fragment variant
- MHC:
-
Major histocompatibility complex
- IL-2:
-
Interleukin-2
- IL-7:
-
Interleukin-7
- IL-15:
-
Interleukin-15
- TNF-α:
-
Tumor necrosis factor-α
- UTD:
-
Untransduced
- IFN-γ:
-
Interferon-γ
- PBMCs:
-
Peripheral blood mononuclear cells
- IVIS:
-
In Vivo Imaging System
- LDH:
-
Lactate dehydrogenase
- TNBC:
-
Triple-negative breast cancer
- TIL:
-
Tumor infiltrated lymphocyte
References
June CH, Riddell SR, Schumacher TN (2015) Adoptive cellular therapy: a race to the finish line. SciTranslMed 7(280):2807–7. https://doi.org/10.1126/scitranslmed.aaa3643
Rosenberg SA, Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348(6230):62–68. https://doi.org/10.1126/science.aaa4967
Maude SL, Frey N, Shaw PA et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517. https://doi.org/10.1056/NEJMoa1407222
Tchou J, Zhao Y, Levine BL et al (2017) Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol Res 5(12):1152–1161. https://doi.org/10.1158/2326-6066.CIR-17-0189
Zhang LN, Song Y, Liu D (2018) CD19 CAR-T cell therapy for relapsed/refractory acute lymphoblastic leukemia: factors affecting toxicities and long-term efficacies. J Hematol Oncol 11(1):41. https://doi.org/10.1186/s13045-018-0593-5
Li J, Li W, Huang K et al (2018) Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol 11(1):22. https://doi.org/10.1186/s13045-018-0568-6
Cubas R, Li M, Chen C et al (2009) Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta 1796(2):309–314. https://doi.org/10.1016/j.bbcan.2009.08.001
Bardia A, Mayer IA, Diamond JR et al (2017) Efficacy and safety of Anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol 35(19):2141–2148. https://doi.org/10.1200/jco.2016.70.8297
Tsukahara Y, Tanaka M, Miyajima A (2011) TROP2 expressed in the trunk of the ureteric duct regulates branching morphogenesis during kidney development. PLoS ONE 6(12):e28607. https://doi.org/10.1371/journal.pone.0028607
Okabe M, Tsukahara Y, Tanaka M et al (2009) Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development 136(11):1951–1960. https://doi.org/10.1242/dev.031369
Zhang B, Yao Y-F (2010) Gelatinous drop-like corneal dystrophy with a novel mutation of TACSTD2 manifested in combination with spheroidal degeneration in a Chinese patient. Mol Vis 16:1570–1575
Wang J, Day R, Dong Y et al (2008) Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol Cancer Ther 7(2):280–285. https://doi.org/10.1158/1535-7163.MCT-07-2003
Tang G, Tang Q, Jia L et al (2018) High expression of TROP2 is correlated with poor prognosis of oral squamous cell carcinoma. Pathol Res Pract 214(10):1606–1612. https://doi.org/10.1016/j.prp.2018.07.017
Trerotola M, Jernigan DL, Liu Q et al (2013) Trop-2 promotes prostate cancer metastasis by modulating beta(1) integrin functions. Cancer Res 73(10):3155–3167. https://doi.org/10.1158/0008-5472.CAN-12-3266
Bignotti E, Todeschini P, Calza S et al (2010) Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur J Cancer 46(5):944–953. https://doi.org/10.1016/j.ejca.2009.12.019
Heist RS, Guarino MJ, Masters G et al (2017) Therapy of advanced non-small-cell lung cancer with an sn-38-anti-trop-2 drug conjugate, sacituzumab Govitecan. J Clin Oncol 35(24):2790–2797. https://doi.org/10.1200/jco.2016.72.1894
Faltas B, Goldenberg DM, Ocean AJ et al (2016) Sacituzumab govitecan, a novel antibody-drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer 14(1):e75–e79. https://doi.org/10.1016/j.clgc.2015.10.002
Starodub AN, Ocean AJ, Shah MA et al (2015) First-in-human trial of a novel anti-trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res 21(17):3870–3878. https://doi.org/10.1158/1078-0432.CCR-14-3321
Fesnak AD, June CH, Levine BL (2016) Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 16(9):566–581. https://doi.org/10.1038/nrc.2016.97
Boleto G, Allanore Y, Avouac J (2018) Targeting costimulatory pathways in systemic sclerosis. Front Immunol 9:2998. https://doi.org/10.3389/fimmu.2018.02998
Kershaw MH, Westwood JA, Darcy PK (2013) Gene-engineered T cells for cancer therapy. Nat Rev Cancer 13(8):525–541. https://doi.org/10.1038/nrc3565
Labanieh L, Majzner RG, Mackall CL (2018) Programming CAR-T cells to kill cancer. Nat Biomed Eng 2(6):377–391. https://doi.org/10.1038/s41551-018-0235-9
Yu S, Li A, Liu Q et al (2017) Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol 10(1):78. https://doi.org/10.1186/s13045-017-0444-9
Sacchetti B, Botticelli A, Pierelli L, et al. CAR-T with License to Kill Solid Tumors in Search of a Winning Strategy. Int J Mol Sci. 2019; https://doi.org/https://doi.org/10.3390/ijms20081903
Croft M (2009) The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol 9(4):271–285. https://doi.org/10.1038/nri2526
van Oosterwijk MF, Juwana H, Arens R et al (2007) CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int Immunol 19(6):713–718. https://doi.org/10.1093/intimm/dxm033
Peperzak V, Veraar EA, Keller AM et al (2010) The Pim kinase pathway contributes to survival signaling in primed CD8+ T cells upon CD27 costimulation. Journal of immunology 185(11):6670–6678. https://doi.org/10.4049/jimmunol.1000159
Dolfi DV, Boesteanu AC, Petrovas C et al (2008) Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. Journal of immunology 180(5):2912–2921. https://doi.org/10.4049/jimmunol.180.5.2912
Song DG, Ye Q, Poussin M et al (2012) CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood 119(3):696–706. https://doi.org/10.1182/blood-2011-03-344275
Goldenberg DM, Cardillo TM, Govindan SV, et al. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 2015;6(26):22496–512. https://doi.org/https://doi.org/10.18632/oncotarget.4318
Kochenderfer JN, Wilson WH, Janik JE et al (2010) Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116(20):4099–4102. https://doi.org/10.1182/blood-2010-04-281931
Pegram HJ, Park JH, Brentjens RJ (2014) CD28z CARs and armored CARs. Cancer J 20(2):127–133. https://doi.org/10.1097/PPO.0000000000000034
Ying Z, He T, Wang X et al (2019) Parallel Comparison of 4–1BB or CD28 Co-stimulated CD19-Targeted CAR-T Cells for B Cell Non-Hodgkin’s Lymphoma. Mol Ther Oncolytics 15:60–68. https://doi.org/10.1016/j.omto.2019.08.002
Kawalekar OU, O’Connor RS, Fraietta JA et al (2016) Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity 44(2):380–390. https://doi.org/10.1016/j.immuni.2016.01.021
Guedan S, Chen X, Madar A et al (2014) ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood 124(7):1070–1080. https://doi.org/10.1182/blood-2013-10-535245
Hendriks J, Xiao Y, Borst J (2003) CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med 198(9):1369–1380. https://doi.org/10.1084/jem.20030916
Adachi K, Kano Y, Nagai T et al (2018) IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol 36(4):346–351. https://doi.org/10.1038/nbt.4086
Ramakrishna V, Sundarapandiyan K, Zhao B et al (2015) Characterization of the human T cell response to in vitro CD27 costimulation with varlilumab. Journal for immunotherapy of cancer 3:37. https://doi.org/10.1186/s40425-015-0080-2
Bardia A, Mayer IA, Vahdat LT et al (2019) Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med 380(8):741–751. https://doi.org/10.1056/NEJMoa1814213
Zhao W, Jia L, Zhang M et al (2019) The killing effect of novel bi-specific Trop2/PD-L1 CAR-T cell targeted gastric cancer. Am J Cancer Res 9(8):1846–1856
Finney HM, Akbar AN, Lawson ADG (2003) Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR chain. J Immunol 172(1):104–113. https://doi.org/10.4049/jimmunol.172.1.104
Croft M (2003) Costimulation of T cells by OX40, 4–1BB, and CD27. Cytokine Growth Factor Rev 14(3–4):265–273. https://doi.org/10.1016/s1359-6101(03)00025-x
Teijeira A, Labiano S, Garasa S et al (2018) Mitochondrial Morphological and Functional Reprogramming Following CD137 (4–1BB) Costimulation. Cancer Immunol Res 6(7):798–811. https://doi.org/10.1158/2326-6066.CIR-17-0767
Bullock TN (2017) Stimulating CD27 to quantitatively and qualitatively shape adaptive immunity to cancer. Curr Opin Immunol 45:82–88. https://doi.org/10.1016/j.coi.2017.02.001
Hombach AA, Heiders J, Foppe M et al (2012) OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology 1(4):458–466. https://doi.org/10.4161/onci.19855
Yang Y, Kohler ME, Chien CD et al (2017) TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aag1209
Acuto O, Michel F (2003) CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol 3(12):939–951. https://doi.org/10.1038/nri1248
Dong H, Buckner A, Prince J et al (2019) Frontline Science: Late CD27 stimulation promotes IL-7Rα transcriptional re-expression and memory T cell qualities in effector CD8(+) T cells. J Leukoc Biol 106(5):1007–1019. https://doi.org/10.1002/jlb.1hi0219-064r
Fry TJ, Mackall CL (2005) The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol 174(11):6571–6576. https://doi.org/10.4049/jimmunol.174.11.6571
Oliveira ML, Akkapeddi P, Ribeiro D et al (2019) IL-7R-mediated signaling in T-cell acute lymphoblastic leukemia: An update. Adv Biol Regul 71:88–96. https://doi.org/10.1016/j.jbior.2018.09.012
McLane LM, Abdel-Hakeem MS, Wherry EJ (2019) CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 37:457–495. https://doi.org/10.1146/annurev-immunol-041015-055318
Roberts DJ, Franklin NA, Kingeter LM et al (2010) Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother 33(8):769–79. https://doi.org/10.1097/CJI.0b013e3181ee238f
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This research was supported by the Science and Technology Department of Guangdong Province (2017A050501010 and 2016A050503023) and Guangzhou Science, Technology and Innovation Commission (201807010042).
Author information
Authors and Affiliations
Contributions
H.Z and C.H designed the study. C.H, Y.M, W.F, Z.Q, Y.B, and H.Z performed experiments, collected and analyzed data. L.Z provided technical support on the mouse model. C.H and H.Z drafted the manuscript. All authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The research was approved by the Zhongshan School of Medicine, Sun Yat-sen University (Guangzhou, China). This research was performed in accordance with the Declaration of Helsinki and according to national and international guidelines. Whole blood from anonymous healthy volunteer donors was obtained from Guangzhou Blood Center, Guangzhou, in which all volunteers had known and confirmed that the blood was going to be used for scientific research. All mouse experiments were approved by and followed to the ethical regulations of IACUC, SYSU.
Consent for publication
All authors agree to publish this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, H., Wei, F., Yin, M. et al. CD27 enhances the killing effect of CAR T cells targeting trophoblast cell surface antigen 2 in the treatment of solid tumors. Cancer Immunol Immunother 70, 2059–2071 (2021). https://doi.org/10.1007/s00262-020-02838-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02838-8