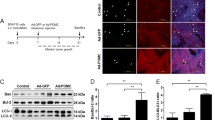
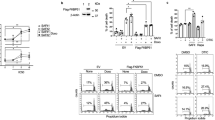
Abstract
Immunotherapy, which has advantages over chemotherapy due to lesser toxicity and higher specificity, is on the rise to treat cancer. Recently, pro-apoptotic glycolipid, ceramide has emerged as a key regulator in cancer immunotherapy. The present study elucidated the potential anti-melanoma efficacy of cell-permeable, exogenous C2 ceramide on cell death and amelioration of tumor microenvironment (TME). We, for the first time, demonstrated that C2 ceramide triggered apoptosis of melanoma cells by augmenting PKCζ along with pro-inflammatory cytokines and signaling factors. C2 ceramide showed a PKCζ-mediated tumor-suppressive role in melanoma without exhibiting hepatotoxicity and nephrotoxicity. Moreover, PKCζ was revealed as one of the key regulators of Akt and ceramide during C2 ceramide-mediated apoptosis. C2 ceramide was effective in repolarization of M2 macrophage phenotype and reduction of angiogenic factors such as VEGF, VEGFR1, VEGFR2, HIF1α. Interestingly, PKCζ knockdown attenuated C2 ceramide-mediated inhibition of melanoma progression. Restoration of the Th1 type TME by C2 ceramide enhanced cytotoxic T cell-mediated killing of melanoma cells. Altogether, the study unraveled that C2 ceramide-induced PKCζ was associated with favorable immune cell functioning in TME leading to melanoma regression. Thus, our findings explored a novel mechanistic insight into C2 ceramide as a promising immunotherapeutic agent in melanoma treatment.






Similar content being viewed by others
Abbreviations
- CM:
-
Conditioned medium
- HGF:
-
Hepatocyte growth factor
- HIF1α:
-
Hypoxia-inducible factor 1-alpha
- MSC:
-
Mesenchymal stromal cells
- PDGF:
-
Platelet-derived growth factor
- PKC:
-
Protein kinase C
- PKCζ:
-
Protein kinase C zeta
- TADC:
-
Tumor-associated dendritic cells
- TAM:
-
Tumor-associated macrophage
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) A global cancer statistics. CA Cancer J Clin 65(2):87–108. https://doi.org/10.3322/caac.21262
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S (2014) Drug resistance in cancer: an overview. Cancers (Basel) 6(3):1769–1792. https://doi.org/10.3390/cancers6031769
Winder M, Virós A (2018) Mechanisms of drug resistance in melanoma. Handb Exp Pharmacol 249:91–108. https://doi.org/10.1007/164_2017_17
Mattia G, Puglisi R, Ascione B, Malorni W, Carè A, Matarrese P (2018) Cell death-based treatments of melanoma:conventional treatments and new therapeutic strategies. Cell Death Dis 9(2):112. https://doi.org/10.1038/s41419-017-0059-7
Bhatia S, Tykodi SS, Thompson JA (2009) Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 23(6):488–496
Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V (2015) Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer 136(10):2352–2360. https://doi.org/10.1002/ijc.29297
Leonardi GC, Falzone L, Salemi R, Zanghì A, Spandidos DA, Mccubrey JA, Candido S, Libra M (2018) Cutaneous melanoma: from pathogenesis to therapy (review). Int J Oncol 52(4):1071–1080. https://doi.org/10.3892/ijo.2018.4287
Ghirelli C, Hagemann T (2013) Targeting immunosuppression for cancer therapy. J Clin Investig 123(6):2355–2357. https://doi.org/10.1172/JCI69999
Cangemi M, Montico B, Faè DA, Steffan A, Dolcetti R (2019) Dissecting the multiplicity of immune effects of immunosuppressive drugs to better predict the risk of de novo malignancies in solid organ transplant patients. Front Oncol 9:160. https://doi.org/10.3389/fonc.2019.00160
Mbeunkui F, Johann DJ Jr (2009) Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol 63(4):571–582. https://doi.org/10.1007/s00280-008-0881-9
Coderch L, López O, de la Maza A, Parra JL (2003) Ceramides and skin function. Am J Clin Dermatol 4(2):107–129. https://doi.org/10.2165/00128071-200304020-00004
Dey R, Majumder N, Bhattacharjee S, Majumdar SB, Banerjee R, Ganguly S, Das P, Majumdar S (2007) Leishmania donovani-induced ceramide as the key mediator of Akt dephosphorylation in murine macrophages: role of protein kinase C zeta and phosphatase. Infect Immun 75(5):2136–2142. https://doi.org/10.1128/IAI.01589-06
Morad SA, Cabot MC (2013) Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 13(1):51–65. https://doi.org/10.1038/nrc3398
Pettus BJ, Chalfant CE, Hannun YA (2002) Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 1585(2–3):114–125. https://doi.org/10.1016/s1388-1981(02)00331-1
Reyland ME (2009) Protein kinase C isoforms: multi-functional regulators of cell life and death. Front Biosci (Landmark Ed) 14:2386–2399
Goodnight J, Mischak H, Mushinski JF (1994) Selective involvement of protein kinase C isozymes in differentiation and neoplastic transformation. Adv Cancer Res 64:159–209
Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M (2007) Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem 282(17):12450–12457. https://doi.org/10.1074/jbc.M700082200
Ahn EH, Schroeder JJ (2010) Induction of apoptosis by sphingosine, sphinganine, and C(2)-ceramide in human colon cancer cells, but not by C(2)-dihydroceramide. Anticancer Res 30(7):2881–2884
Henry B, Möller C, Dimanche-Boitrel MT, Gulbins E, Becker KA (2013) Targeting the ceramide system in cancer. Cancer Lett 332(2):286–294. https://doi.org/10.1016/j.canlet.2011.07.010
Pritzl CJ, Seo YJ, Xia C, Vijayan M, Stokes ZD, Hahm BA (2015) ceramide analogue stimulates dendritic cells to promote T cell responses upon virus infections. J Immunol 194(9):4339–4349. https://doi.org/10.4049/jimmunol.1402672
Kurinna SM, Tsao CC, Nica AF, Jiffar T, Ruvolo PP (2004) Ceramide promotes apoptosis in lung cancer-derived A549 cells by a mechanism involving c-Jun NH2-terminal kinase. Cancer Res 64(21):7852–7856. https://doi.org/10.1158/0008-5472.CAN-04-1552
Stover T, Kester M (2003) Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J Pharmacol Exp Ther 307(2):468–475. https://doi.org/10.1124/jpet.103.054056
Halder K, Banerjee S, Bose A, Majumder S, Majumdar S (2014) Overexpressed PKCδ downregulates the expression of PKCα in B16F10 melanoma: induction of apoptosis by PKCδ via ceramide generation. PLoS ONE 9(3):e91656. https://doi.org/10.1371/journal.pone.0091656
Ghosh S, Jawed JJ, Halder K, Banerjee S, Chowdhury BP, Saha A, Juin SK, Majumdar SB, Bose A, Baral R, Majumdar S (2018) TNFα mediated ceramide generation triggers cisplatin induced apoptosis in B16F10 melanoma in a PKCδ independent manner. Oncotarget 9(102):37627–37646. https://doi.org/10.18632/oncotarget.26478
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76(9):4350–4354. https://doi.org/10.1073/pnas.76.9.4350
Liu H, Zheng H, Duan Z, Hu D, Li M, Liu S, Li Z, Deng X, Wang Z, Tang M, Shi Y, Yi W, Cao Y (2009) LMP1-augmented kappa intron enhancer activity contributes to upregulation expression of Ig kappa light chain via NF-kappaB and AP-1 pathways in nasopharyngeal carcinoma cells. Mol Cancer 8:92. https://doi.org/10.1186/1476-4598-8-92
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159. https://doi.org/10.1006/abio.1987.9999
Mischak H, Pierce JH, Goodnight J, Kazanietz MG, Blumberg PM, Mushinski JF (1993) Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-alpha and -delta and not by protein kinase C-beta II, -epsilon, -zeta, and -eta. J Biol Chem 268(27):20110–20115
Banerjee S, Halder K, Bose A, Bhattacharya P, Gupta G, Karmahapatra S, Das S, Chaudhuri S, Bhattacharyya MS, Majumdar S (2011) TLR signaling-mediated differential histone modification at IL-10 and IL-12 promoter region leads to functional impairments in tumor-associated macrophages. Carcinogenesis 32(12):1789–1797. https://doi.org/10.1093/carcin/bgr208
Berhanu A, Huang J, Alber SM, Watkins SC, Storkus WJ (2006) Combinational FLt3 ligand and granulocyte macrophage colony-stimulating factor treatment promotes enhanced tumor infiltration by dendritic cells and antitumor CD8(+) T-cell cross-priming but is ineffective as a therapy. Cancer Res 66(9):4895–4903. https://doi.org/10.1158/0008-5472.CAN-05-2384
Yang M, McKay D, Pollard JW, Lewis CE (2018) Diverse functions of macrophages in different tumor microenvironments. Cancer Res 78(19):5492–5503. https://doi.org/10.1158/0008-5472.CAN-18-1367
Caux C, Ramos RN, Prendergast GC, Bendriss-Vermare N, Ménétrier-Caux C (2016) A milestone review on how macrophages affect tumor growth. Cancer Res 76(22):6439–6442. https://doi.org/10.1158/0008-5472.CAN-16-2631
Jeong H, Kim S, Hong BJ, Lee CJ, Kim YE, Bok S, Oh JM, Gwak SH, Yoo MY, Lee MS, Chung SJ, Defrêne J, Tessier P, Pelletier M, Jeon H, Roh TY, Kim B, Kim KH, Ju JH, Kim S, Lee YJ, Kim DW, Kim IH, Kim HJ, Park JW, Lee YS, Lee JS, Cheon GJ, Weissman IL, Chung DH, Jeon YK, Ahn GO (2019) Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res 79(4):795–806. https://doi.org/10.1158/0008-5472.CAN-18-2545
Fridman WH, Pagès F, Sautès-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12(4):298–306. https://doi.org/10.1038/nrc3245
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K, Li X, Xiong W, Li G, Zeng Z, Guo C (2017) Role of tumor microenvironment in tumorigenesis. J Cancer 8(5):761–773. https://doi.org/10.7150/jca.17648
Wanderley CW, Colón DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA, Pereira JA, Silva CM, Silva CR, Silva RL, Speck-Hernandez CA, Mota JM, Alves-Filho JC, Lima-Junior RC, Cunha TM, Cunha FQ (2018) Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res 78(20):5891–5900. https://doi.org/10.1158/0008-5472.CAN-17-3480
Elias EG, Hasskamp JH, Sharma BK (2010) Cytokines and growth factors expressed by human cutaneous melanoma. Cancers (Basel) 2(2):794–808. https://doi.org/10.3390/cancers2020794
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125(Pt 23):5591–5596. https://doi.org/10.1242/jcs.116392
Banerjee S, Halder K, Ghosh S, Bose A, Majumdar S (2015) The combination of a novel immunomodulator with a regulatory T cell suppressing antibody (DTA-1) regress advanced stage B16F10 solid tumor by repolarizing tumor associated macrophages in situ. Oncoimmunology 4(3):e995559. https://doi.org/10.1080/2162402X.2014.995559
Halder K, Banerjee S, Ghosh S, Bose A, Das S, Chowdhury BP, Majumdar S (2017) Mycobacterium indicus pranii (Mw) inhibits invasion by reducing matrix metalloproteinase (MMP-9) via AKT/ERK-1/2 and PKCα signaling: a potential candidate in melanoma cancer therapy. Cancer Biol Ther 18(11):850–862. https://doi.org/10.1080/15384047.2015.1078024
Payne AW, Pant DK, Pan TC, Chodosh LA (2014) Ceramide kinase promotes tumor cell survival and mammary tumor recurrence. Cancer Res 74(21):6352–6363. https://doi.org/10.1158/0008-5472.CAN-14-1292
van Vlerken LE, Duan Z, Seiden MV, Amiji MM (2007) Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res 67(10):4843–4850. https://doi.org/10.1158/0008-5472.CAN-06-1648
Flowers M, Fabriás G, Delgado A, Casas J, Abad JL, Cabot MC (2012) C6-ceramide and targeted inhibition of acid ceramidase induce synergistic decreases in breast cancer cell growth. Breast Cancer Res Treat 133(2):447–458. https://doi.org/10.1007/s10549-011-1768-8
Liu Z, Xia Y, Li B, Xu H, Wang C, Liu Y, Li Y, Li C, Gao N, Li L (2014) Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca(2+) homeostasis in human adenoid cystic carcinoma cells. Cell Biosci 4:71. https://doi.org/10.1186/2045-3701-4-71
Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG (2003) Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem 278(36):33753–33762. https://doi.org/10.1074/jbc.M303313200
Isakov N (2018) Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin Cancer Biol 48:36–52. https://doi.org/10.1016/j.semcancer.2017.04.012
Zhang P, Fu C, Hu Y, Dong C, Song Y, Song E (2015) C6-ceramide nanoliposome suppresses tumor metastasis by eliciting PI3K and PKCζ tumor-suppressive activities and regulating integrin affinity modulation. Sci Rep 5:9275. https://doi.org/10.1038/srep09275
Fan HH, Li L, Zhang YM, Yang J, Li MC, Zeng FY, Deng F (2017) PKCζ in prostate cancer cells represses the recruitment and M2 polarization of macrophages in the prostate cancer microenvironment. Tumour Biol 39(6):1010428317701442. https://doi.org/10.1177/1010428317701442
Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, Bielawska A, Obeid LM, Hannun YA (2002) Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem 277(15):12960–12969. https://doi.org/10.1074/jbc.M110699200
Bourbon NA, Sandirasegarane L, Kester M (2002) Ceramide-induced inhibition of Akt is mediated through protein kinase C zeta: implications for growth arrest. J Biol Chem 277(5):3286–3292. https://doi.org/10.1074/jbc.M110541200
Bourbon NA, Yun J, Kester M (2000) Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem 275(45):35617–35623. https://doi.org/10.1074/jbc.M007346200
Carpinteiro A, Becker KA, Japtok L, Hessler G, Keitsch S, Požgajovà M, Schmid KW, Adams C, Müller S, Kleuser B, Edwards MJ, Grassmé H, Helfrich I, Gulbins E (2015) Regulation of hematogenous tumor metastasis by acid sphingomyelinase. EMBO Mol Med 7(6):714–734. https://doi.org/10.15252/emmm.201404571
Osawa Y, Suetsugu A, Matsushima-Nishiwaki R, Yasuda I, Saibara T, Moriwaki H, Seishima M, Kozawa O (2013) Liver acid sphingomyelinase inhibits growth of metastatic colon cancer. J Clin Investig 123(2):834–843. https://doi.org/10.1172/JCI65188
Acknowledgements
We are thankful to the Director, Bose Institute (Kolkata, India), for his cooperation during the study. We thank members of the Central Instrument Facility for their assistance and Mr. Prabal Gupta for technical support.
Funding
The work was supported by the funds from the Council of Scientific and Industrial Research (CSIR), India [Grant no. 09/015(0475)2015-EMR-1].
Author information
Authors and Affiliations
Contributions
SG designed and performed the experiments, analyzed the data, and wrote the paper; SKJ performed the experiments and analyzed the data; PN performed few experiments; SBM, AB, RNB, and PCS helped in performing some of the experiments; SM designed the project, analyzed the data, and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments involving animals in this study were carried out in strict accordance with guidelines established by the Institutional Animal Ethical Committee (IAEC), Bose Institute, Kolkata, India. All the experimental plan of work consisting of animals was approved by IAEC, Bose Institute and CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Govt. of India (Reg. no. 1796/GO/EReBiBt/S/14/CPCSEA; Approval no. IAEC/BI/18/2015).
Animal source
Female C57BL/6 mice were procured from the Central Animal House and Research Facility of Bose Institute (Kolkata, India).
Cell line authentication
B16F10 murine melanoma cells, A375 human melanoma cells, RAW264.7 murine macrophages, and THP1-derived macrophages, a non-adherent human monocyte, were procured from the National Centre for Cell Sciences, (Pune, India). The cell lines were authenticated by the vendor and the cells were used as provided by the vendor.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghosh, S., Juin, S.K., Nandi, P. et al. PKCζ mediated anti-proliferative effect of C2 ceramide on neutralization of the tumor microenvironment and melanoma regression. Cancer Immunol Immunother 69, 611–627 (2020). https://doi.org/10.1007/s00262-020-02492-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02492-0