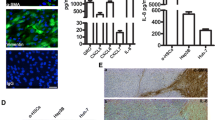
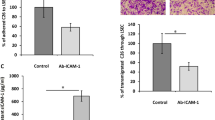
Abstract
Hepatic stellate cells (HSCs) are important stromal cells and pivotal mediators involved in the pathogenesis and immunosuppression of hepatocellular carcinoma (HCC). The liver has been demonstrated to be a site for accumulation of tumor-induced myeloid-derived suppressor cells (MDSCs). We previously reported that HSCs induced an increase in the number of MDSCs in HCC. However, how MDSCs are recruited in HCC remains largely unclear. In the present study, we found that HSC-conditioned medium (HSC-CM) induced bone marrow-derived cell and splenocyte migration, especially MDSC migration. Using chemokine-neutralizing antibodies and chemokine receptor inhibitors, we found that HSCs promoted MDSC migration through the SDF-1/CXCR4 axis. Subsequently, we used an orthotopic mouse liver tumor model to determine how HSCs mediated MDSC migration to HCC in vivo. The in vivo results indicated that pretreatment of MDSCs with a CXCR4 inhibitor or injection with SDF-1-knocked down HSCs inhibited MDSC migration to the spleen and liver of the tumor-bearing mice. Together, our findings indicate a central role for HSCs in MDSC migration mediated by the SDF-1/CXCR4 axis, thus revealing a potentially effective approach for modulating the tumor microenvironment by targeting HSCs in HCC.





Similar content being viewed by others
Abbreviations
- C3:
-
Complement component 3
- CCL/CCR:
-
CC chemokine ligand/chemokine receptor
- CXCL/CXCR:
-
CXC chemokine ligand/chemokine receptor
- DiR:
-
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide
- G-MDSCs:
-
Granulocytic myeloid-derived suppressor cells
- HCC:
-
Hepatocellular carcinoma
- HSC-CM:
-
HSC conditioned medium
- HSCs:
-
Hepatic stellate cells
- IL-1β:
-
Interleukin-1 beta
- IL-8:
-
Interleukin-8
- IVIS:
-
In vivo imaging system
- MDSCs:
-
Myeloid-derived suppressor cells
- Mo-MDSCs:
-
Monocytic myeloid-derived suppressor cells
- PD-L1:
-
Programmed death ligand 1
- RBC:
-
Red blood cells
- SDF-1:
-
Stromal cell-derived factor 1
References
Zhang H, Li Z, Wang L, Tian G, Tian J, Yang Z, Cao G, Zhou H, Zhao L, Wu Z, Yin Z (2017) Critical role of myeloid-derived suppressor cells in tumor-induced liver immune suppression through inhibition of NKT cell function. Front Immunol 8:129. https://doi.org/10.3389/fimmu.2017.00129
Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F (2009) Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50(3):799–807. https://doi.org/10.1002/hep.23054
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9(3):162–174. https://doi.org/10.1038/nri2506
Schmid MC, Varner JA (2012) Myeloid cells in tumor inflammation. Vasc Cell 4(1):14. https://doi.org/10.1186/2045-824X-4-14
Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Can Res 70(1):68–77. https://doi.org/10.1158/0008-5472.CAN-09-2587
Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL (2008) Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13(1):23–35. https://doi.org/10.1016/j.ccr.2007.12.004
Schmid MC, Avraamides CJ, Foubert P, Shaked Y, Kang SW, Kerbel RS, Varner JA (2011) Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer Res 71(22):6965–6975. https://doi.org/10.1158/0008-5472.CAN-11-0588
Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, Fein M, Rasch MG, Tan YX, Qiu J, Park J, Sinha P, Bissell MJ, Frengen E, Werb Z, Egeblad M (2012) Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 21(4):488–503. https://doi.org/10.1016/j.ccr.2012.02.017
Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G (2008) HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13(3):206–220. https://doi.org/10.1016/j.ccr.2008.01.034
Ilkovitch D, Lopez DM (2009) The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res 69(13):5514–5521. https://doi.org/10.1158/0008-5472.CAN-08-4625
Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, Hewitt S, Zender L, Kapoor V, Felsher DW, Manns MP, Korangy F, Greten TF (2013) Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J Hepatol 59(5):1007–1013. https://doi.org/10.1016/j.jhep.2013.06.010
Umansky V, Sevko A (2013) Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron 6(2):169–177. https://doi.org/10.1007/s12307-012-0126-7
Tsuchida T, Friedman SL (2017) Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 14(7):397–411. https://doi.org/10.1038/nrgastro.2017.38
Thompson AI, Conroy KP, Henderson NC (2015) Hepatic stellate cells: central modulators of hepatic carcinogenesis. BMC gastroenterology 15:63. https://doi.org/10.1186/s12876-015-0291-5
Aimaiti Y, Jin X, Shao Y, Wang W, Li D (2019) Hepatic stellate cells regulate hepatic progenitor cells differentiation via the TGF-beta1/Jagged1 signaling axis. J Cell Physiol 234(6):9283–9296. https://doi.org/10.1002/jcp.27609
Ji F, Wang K, Zhang Y, Mao XL, Huang Q, Wang J, Ye L, Li Y (2019) MiR-542-3p controls hepatic stellate cell activation and fibrosis via targeting BMP-7. J Cell Biochem 120(3):4573–4581. https://doi.org/10.1002/jcb.27746
Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S (2004) Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 40(6):1312–1321. https://doi.org/10.1002/hep.20488
Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, Stolz DB, Chen L, Fung JJ, Lu L, Qian S (2006) In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology 44(5):1171–1181. https://doi.org/10.1002/hep.21379
Chou HS, Hsieh CC, Yang HR, Wang L, Arakawa Y, Brown K, Wu Q, Lin F, Peters M, Fung JJ, Lu L, Qian S (2011) Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology 53(3):1007–1019. https://doi.org/10.1002/hep.24162
Hsieh CC, Chou HS, Yang HR, Lin F, Bhatt S, Qin J, Wang L, Fung JJ, Qian S, Lu L (2013) The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood 121(10):1760–1768. https://doi.org/10.1182/blood-2012-06-440214
Hochst B, Schildberg FA, Sauerborn P, Gabel YA, Gevensleben H, Goltz D, Heukamp LC, Turler A, Ballmaier M, Gieseke F, Muller I, Kalff J, Kurts C, Knolle PA, Diehl L (2013) Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J Hepatol 59(3):528–535. https://doi.org/10.1016/j.jhep.2013.04.033
Zhao W, Zhang L, Yin Z, Su W, Ren G, Zhou C, You J, Fan J, Wang X (2011) Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int J Cancer 129(11):2651–2661. https://doi.org/10.1002/ijc.25920
Zhao W, Zhang L, Xu Y, Zhang Z, Ren G, Tang K, Kuang P, Zhao B, Yin Z, Wang X (2014) Hepatic stellate cells promote tumor progression by enhancement of immunosuppressive cells in an orthotopic liver tumor mouse model. Lab Invest 94(2):182–191. https://doi.org/10.1038/labinvest.2013.139
Xu Y, Zhao W, Xu J, Li J, Hong Z, Yin Z, Wang X (2016) Activated hepatic stellate cells promote liver cancer by induction of myeloid-derived suppressor cells through cyclooxygenase-2. Oncotarget 7(8):8866–8878. https://doi.org/10.18632/oncotarget.6839
Zhao W, Su W, Kuang P, Zhang L, Liu J, Yin Z, Wang X (2012) The role of hepatic stellate cells in the regulation of T-cell function and the promotion of hepatocellular carcinoma. Int J Oncol 41(2):457–464. https://doi.org/10.3892/ijo.2012.1497
Xu Y, Zhao W, Wu D, Xu J, Lin S, Tang K, Yin Z, Wang X (2014) Isolation of myeloid-derived suppressor cells subsets from spleens of orthotopic liver cancer-bearing mice by fluorescent-activated and magnetic-activated cell sorting: similarities and differences. Int J Clin Exp Pathol 7(11):7545–7553
Zollo M, Di Dato V, Spano D, De Martino D, Liguori L, Marino N, Vastolo V, Navas L, Garrone B, Mangano G, Biondi G, Guglielmotti A (2012) Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin Exp Metastasis 29(6):585–601. https://doi.org/10.1007/s10585-012-9473-5
Karin N, Razon H (2018) The role of CCR28 in directing the mobilization and biological function of CD11b(+)Gr1(+)Ly6C(low) polymorphonuclear myeloid cells in cancer. Cancer Immunol Immunother CII 67(12):1949–1953. https://doi.org/10.1007/s00262-018-2245-6
Liepelt A, Tacke F (2016) Stromal cell-derived factor-1 (SDF-1) as a target in liver diseases. Am J Physiol Gastrointest Liver Physiol 311(2):G203–209. https://doi.org/10.1152/ajpgi.00193.2016
Shou D, Wen L, Song Z, Yin J, Sun Q, Gong W (2016) Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget 7(39):64505–64511. https://doi.org/10.18632/oncotarget.11352
Zhao W, Xu Y, Xu J, Wu D, Zhao B, Yin Z, Wang X (2015) Subsets of myeloid-derived suppressor cells in hepatocellular carcinoma express chemokines and chemokine receptors differentially. Int Immunopharmacol 26(2):314–321. https://doi.org/10.1016/j.intimp.2015.04.010
Xia Y, Chen R, Ye SL, Sun R, Chen J, Zhao Y (2011) Inhibition of T-cell responses by intratumoral hepatic stellate cells contribute to migration and invasion of hepatocellular carcinoma. Clin Exp Metastasis 28(7):661–674. https://doi.org/10.1007/s10585-011-9399-3
Schildberg FA, Sharpe AH, Turley SJ (2015) Hepatic immune regulation by stromal cells. Curr Opin Immunol 32:1–6. https://doi.org/10.1016/j.coi.2014.10.002
Ji J, Eggert T, Budhu A, Forgues M, Takai A, Dang H, Ye Q, Lee JS, Kim JH, Greten TF, Wang XW (2015) Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology 62(2):481–495. https://doi.org/10.1002/hep.27822
Damianakou C, Tsokas C (1987) Clinical and technical characteristics of alginate hydrocolloid. Hellenika Stomatologika Chronika Hell Stomatol Ann 31(1):63–68
Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P (2011) PGE(2)-induced CXCL12 production and CXCR36 expression controls the accumulation of human MDSCs in ovarian cancer environment. Can Res 71(24):7463–7470. https://doi.org/10.1158/0008-5472.CAN-11-2449
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475(7355):222–225. https://doi.org/10.1038/nature10138
Umansky V, Blattner C, Gebhardt C, Utikal J (2017) CCR38 in recruitment and activation of myeloid-derived suppressor cells in melanoma. Cancer Immunol Immunother CII 66(8):1015–1023. https://doi.org/10.1007/s00262-017-1988-9
Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, Kanojia D, Pituch KC, Qiao J, Pytel P, Han Y, Wu M, Zhang L, Horbinski CM, Ahmed AU, Lesniak MS (2016) CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res 76(19):5671–5682. https://doi.org/10.1158/0008-5472.CAN-16-0144
Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL (2014) Disruption of CXCR1-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med 6(237):237ra267. https://doi.org/10.1126/scitranslmed.3007974
Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN (2013) CXCR41-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 24(5):631–644. https://doi.org/10.1016/j.ccr.2013.10.009
Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K (2008) Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111(12):5457–5466. https://doi.org/10.1182/blood-2008-01-136895
Li Y, Lu L, Qian S, Fung JJ, Lin F (2016) Hepatic stellate cells directly inhibit B cells via programmed death-ligand 1. J Immunol 196(4):1617–1625. https://doi.org/10.4049/jimmunol.1501737
Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother CII 59(10):1593–1600. https://doi.org/10.1007/s00262-010-0855-8
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V (2012) Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12(4):253–268. https://doi.org/10.1038/nri3175
Fruci D, Lo Monaco E, Cifaldi L, Locatelli F, Tremante E, Benevolo M, Giacomini P (2013) T and NK cells: two sides of tumor immunoevasion. J Transl Med 11:30. https://doi.org/10.1186/1479-5876-11-30
Pyzer AR, Cole L, Rosenblatt J, Avigan DE (2016) Myeloid-derived suppressor cells as effectors of immune suppression in cancer. Int J Cancer 139(9):1915–1926. https://doi.org/10.1002/ijc.30232
Wang Y, Schafer CC, Hough KP, Tousif S, Duncan SR, Kearney JF, Ponnazhagan S, Hsu HC, Deshane JS (2018) Myeloid-derived suppressor cells impair B cell responses in lung cancer through IL-7 and STAT5. J Immunol 201(1):278–295. https://doi.org/10.4049/jimmunol.1701069
Stiff A, Trikha P, Mundy-Bosse B, McMichael E, Mace TA, Benner B, Kendra K, Campbell A, Gautam S, Abood D, Landi I, Hsu V, Duggan M, Wesolowski R, Old M, Howard JH, Yu L, Stasik N, Olencki T, Muthusamy N, Tridandapani S, Byrd JC, Caligiuri M, Carson WE (2018) Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing Fc receptor-mediated natural killer cell function. Clin Cancer Res 24(8):1891–1904. https://doi.org/10.1158/1078-0432.CCR-17-0691
Funding
This work was supported by grants from the National Nature Science Foundation of China (81602500 and 81572335); Outstanding Youth Science Research Personnel Training Plan of Fujian Province Colleges and Universities (2017); and Doctoral Start-up Foundation of Xiamen Medical College (K2016-07).
Author information
Authors and Affiliations
Contributions
YX performed most of the experiments, analyzed the data, and wrote the manuscript. FF, HJ, XZ, LH, and XY assisted with and/or performed some of the experiments and revised the manuscript. WZ contributed to the design of the experiments and analysis and interpretation of the data, and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal experimental protocols were performed in compliance with the Guidelines for the Institutional Animal Care and Use Committee of Xiamen University. (the date of animal research approval: 2015-02-26).
Animal source
BALB/c (H-2d, haplotype) mice aged 8–12 weeks were purchased from the National Rodent Laboratory Animal Resources, Shanghai, China.
Cell authentication
Not applicable because murine primary HSCs and MDSCs and murine HCC cell line H22 cells were used, which are lacking of short tandem repeat data. The H22 cells were purchased from Shanghai Cell Bank, Chinese Academy of Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, Y., Fang, F., Jiao, H. et al. Activated hepatic stellate cells regulate MDSC migration through the SDF-1/CXCR4 axis in an orthotopic mouse model of hepatocellular carcinoma. Cancer Immunol Immunother 68, 1959–1969 (2019). https://doi.org/10.1007/s00262-019-02414-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02414-9