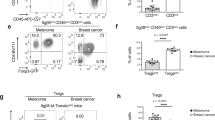
Abstract
Various immune cells are recruited in the tumor microenvironment. It is well established that cellular immune responses, such as cytotoxic or suppressive activities, play an important role in regulating tumor growth and metastasis. However, the contribution of humoral immune responses against tumors is poorly understood. Fc receptors constitute critical elements for the up- or downregulation of immune responses through immune complexes. Here, we examined the potential role of the inhibitory Fc receptor, Fcγ receptor IIB (FcγRIIB), in tumor immunity using a mouse model. Our findings indicated that tumor-specific antibodies are induced in tumor-bearing mice and control tumor immunity. FcγRIIB deletion significantly improved both cellular and humoral immunity against tumors and delayed tumor growth. These findings indicated that spontaneous antibodies against tumors create a suppressive tumor microenvironment through FcγRIIB signaling, thus suggesting an attractive therapeutic target for cancer immunotherapy.






Similar content being viewed by others
Abbreviations
- BMDMs:
-
Bone marrow-derived macrophages
- FcRs:
-
Fc receptors
- FcγRIIB:
-
Fcγ receptor IIB
- gMDSCs:
-
Granulocytic MDSCs
- HT:
-
Heterozygous
- KO:
-
Knockout
- LLC:
-
Lewis lung carcinoma
- mMDSCs:
-
Monocytic myeloid-derived suppressor cells
- OVA:
-
Ovalbumin
- WT:
-
Wild-type
References
Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14:1014–1022
Zitvogel L, Tesniere A, Kroemer G (2006) Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 6:715–727
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (New York, N.Y.) 331:1565–1570
van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM (2003) The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol 82:539–548
Joyce JA, Fearon DT (2015) T cell exclusion, immune privilege, and the tumor microenvironment. Science (New York, N.Y.) 348:74–80
Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T (2003) A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res 63:4095–4100
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19:1423–1437
Nimmerjahn F, Ravetch JV (2008) Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 8:34–47
Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM, Ravetch JV (2014) Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol 15:707–716
DiLillo DJ, Ravetch JV (2015) Fc-receptor interactions regulate both cytotoxic and immunomodulatory therapeutic antibody effector functions. Cancer Immunol Res 3:704–713
Bournazos S, Wang TT, Dahan R, Maamary J, Ravetch JV (2017) Signaling by antibodies: recent progress. Annu Rev Immunol 35:285–311
Nimmerjahn F, Ravetch JV (2006) Fcgamma receptors: old friends and new family members. Immunity 24:19–28
Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV (2002) Genetic modifiers of systemic lupus erythematosus in Fc gamma RIIB(−/−) mice. J Exp Med 195:1167–1174
Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV (1999) Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med 189:179–185
Jiang Y, Hirose S, Abe M, Sanokawa-Akakura R, Ohtsuji M, Mi X, Li N, Xiu Y, Zhang D, Shirai J, Hamano Y, Fujii H, Shirai T (2000) Polymorphisms in IgG Fc receptor IIB regulatory regions associated with autoimmune susceptibility. Immunogenetics 51:429–435
McGaha TL, Karlsson MC, Ravetch JV (2008) FcgammaRIIB deficiency leads to autoimmunity and a defective response to apoptosis in Mrl–MpJ mice. J Immunol 180:5670–5679
Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV (1996) Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature 379:346–349
Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, Ravetch JV, Takai T (1999) Deletion of fc gamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J Exp Med 189:187–194
Kobayashi N, Hong C, Klinman DM, Shirota H (2013) Oligodeoxynucleotides expressing polyguanosine motifs promote antitumor activity through the upregulation of IL-2. J Immunol 190:1882–1889
Shirota Y, Shirota H, Klinman DM (2012) Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol 188:1592–1599
Ito SE, Shirota H, Kasahara Y, Saijo K, Ishioka C (2017) IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model. Cancer Immunol Immunother CII 66:1485–1496
Warren MK, Vogel SN (1985) Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J Immunol 134:982–989
Benvenuto M, Mattera R, Masuelli L, Tresoldi I, Giganti MG, Frajese GV, Manzari V, Modesti A, Bei R (2017) The crossroads between cancer immunity and autoimmunity: antibodies to self antigens. Front Biosci (Landmark Ed) 22:1289–1329
Reuschenbach M, von Knebel Doeberitz M, Wentzensen N (2009) A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother CII 58:1535–1544
Zaenker P, Gray ES, Ziman MR (2016) Autoantibody production in cancer-the humoral immune response toward autologous antigens in cancer patients. Autoimmun Rev 15:477–483
Shirota H, Klinman DM, Ito SE, Ito H, Kubo M, Ishioka C (2017) IL4 from T follicular helper cells downregulates antitumor immunity. Cancer Immunol Res 5:61–71
Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM, Kumari S, Kelly RL, Kwan BH, Abraham W, Hu K, Mehta NK, Kauke MJ, Suh H, Cochran JR, Lauffenburger DA, Wittrup KD, Irvine DJ (2016) Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med 22:1402–1410
Pearce OM, Laubli H, Verhagen A, Secrest P, Zhang J, Varki NM, Crocker PR, Bui JD, Varki A (2014) Inverse hormesis of cancer growth mediated by narrow ranges of tumor-directed antibodies. Proc Natl Acad Sci USA 111:5998–6003
Gul N, van Egmond M (2015) Antibody-dependent phagocytosis of tumor cells by macrophages: a potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res 75:5008–5013
Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV (2015) FcgammaRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell 28:285–295
Roghanian A, Teige I, Martensson L, Cox KL, Kovacek M, Ljungars A, Mattson J, Sundberg A, Vaughan AT, Shah V, Smyth NR, Sheth B, Chan HT, Li ZC, Williams EL, Manfredi G, Oldham RJ, Mockridge CI, James SA, Dahal LN, Hussain K, Nilsson B, Verbeek JS, Juliusson G, Hansson M, Jerkeman M, Johnson PW, Davies A, Beers SA, Glennie MJ, Frendeus B, Cragg MS (2015) Antagonistic human FcgammaRIIB (CD32B) antibodies have anti-tumor activity and overcome resistance to antibody therapy in vivo. Cancer Cell 27:473–488
Takai T (2002) Roles of Fc receptors in autoimmunity. Nat Rev Immunol 2:580–592
van Montfoort N, t Hoen PA, Mangsbo SM, Camps MG, Boross P, Melief CJ, Ossendorp F, Verbeek JS (2012) Fcgamma receptor IIB strongly regulates Fc gamma receptor-facilitated T cell activation by dendritic cells. J Immunol 189:92–101
Muller AJ, Scherle PA (2006) Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer 6:613–625
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8:1069–1086
Williams EL, Tutt AL, Beers SA, French RR, Chan CH, Cox KL, Roghanian A, Penfold CA, Butts CL, Boross P, Verbeek JS, Cragg MS, Glennie MJ (2013) Immunotherapy targeting inhibitory Fcγ receptor IIB (CD32b) in the mouse is limited by monoclonal antibody consumption and receptor internalization. J Immunol 19:4130–4140
Daeron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S, Fridman WH (1995) The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity 3:635–646
Nimmerjahn F, Ravetch JV (2005) Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science (New York, N.Y.) 310:1510–1512
Acknowledgements
The authors thank Dr. Toshiyuki Takai for kindly providing FcγRIIB KO mice and a critical review of this manuscript. The authors would like to thank Enago (http://www.enago.jp) for the English language review.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant nos. 25430103 and 16K07106, Japan.
Author information
Authors and Affiliations
Contributions
HS and CI conceived the concept. HS and YK designed the experiments. YK and SU performed the in vitro and in vivo experiments. HS, YK and SU analyzed the data. HS and YK wrote the paper.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Institutional Committee for the Use and Care of Laboratory Animals of Tohoku University. Animal research approval number: 2017-171-1. All in vivo experiments were conducted in strict accordance with good animal practice and complied with ethics committee guidelines of Tohoku University.
Conflict of interest
The authors declare that they have no conflicts of interests.
Animal source
C57Bl/6 mice were obtained from Japan SLC (Hamamatsu, Japan). FcγRIIB KO mice were provided by Takai et al. (Department of Experimental Immunology, Tohoku University, Sendai, Japan).
Cell line authentication
The following cell lines were purchased from American Type Culture collection (Manassas, VA): E.G7, LLC, TC-1 and B16 melanoma. No cell line authentication was necessary.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kasahara, Y., Shirota, H., Umegaki, S. et al. Contribution of Fcγ receptor IIB to creating a suppressive tumor microenvironment in a mouse model. Cancer Immunol Immunother 68, 1769–1778 (2019). https://doi.org/10.1007/s00262-019-02413-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02413-w