Abstract
Introduction
Ulcerated melanomas may have a unique biology and microenvironment. We test whether markers of immune infiltration correlate with clinical outcome in ulcerated compared to non-ulcerated primary melanoma tumors.
Methods
Sixty-two stage II–III cutaneous melanomas, 32 ulcerated and 30 non-ulcerated, were analyzed for tumor-infiltrating lymphocytes (TILs). Immunohistochemistry (IHC) was performed for CD2, a marker previously shown to correlate with overall survival (OS) and recurrence-free survival (RFS) in this patient population. IHC using antibody, VE1, to BRAF V600E was also performed on a subset of 41 tumors to assess the relationship of BRAF mutation to immune markers.
Results
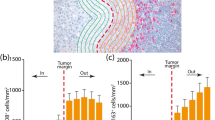
We found, using Cox regression models, that the presence of TILs was associated with improved OS (p = 0.034) and RFS (p = 0.002) in ulcerated melanoma tumors, but not in non-ulcerated melanoma (p = 0.632, 0.416). CD2 expression also was correlated with improved OS (p = 0.021) and RFS (p = 0.001) in ulcerated melanoma, but no relationship was seen in non-ulcerated melanoma (p = 0.427, 0.682). In this small population, BRAF status did not correlate with TILs or CD2+ count.
Conclusion
Our data show that immune markers including TILs and CD2 count correlate more closely with survival in ulcerated melanomas than that in non-ulcerated melanomas. We propose that immune biomarkers may be particularly relevant to ulcerated, as compared to non-ulcerated, melanomas and that this merits study in larger populations.



Similar content being viewed by others
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- Ang-2:
-
Angiopoietin 2
- EORTC:
-
European Organisation for Research and Treatment of Cancer
- FFPE:
-
Formalin fixed paraffin embedded
- GMC:
-
Geisinger Medical Center
- HPF:
-
High-powered field
- IHC:
-
Immunohistochemistry
- IRB:
-
Institutional review board
- ISMMS:
-
Icahn School of Medicine at Mount Sinai
- LFA-3:
-
Lymphocyte function-associated antigen-3
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
- TILs:
-
Tumor-infiltrating lymphocytes
- TNBC:
-
Triple-negative breast cancer
References
Allen AC, Spitz S (1953) Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer 6:1–45
Eggermont AM, Spatz A, Lazar V, Robert C (2012) Is ulceration in cutaneous melanoma just a prognostic and predictive factor or is ulcerated melanoma a distinct biologic entity? Curr Opin Oncol 24:137–140. doi:10.1097/CCO.0b013e32834fcb0d
McMasters KM, Edwards MJ, Ross MI et al (2010) Ulceration as a predictive marker for response to adjuvant interferon therapy in melanoma. Ann Surg. 252:460–465; discussion 465–466. doi: 10.1097/SLA.0b013e3181f20bb1
Suciu S, Ives N, Eggermont AM, Kirkwood JM, Lorigan P, Markovic S, Garbe C, Wheatley K (2014) Predictive importance of ulceration on the efficacy of adjuvant interferon-a (IFN): AN individual patient data (IPD) meta-analysis of 15 randomized trials in more than 7,500 melanoma patients (pts). J Clin Oncol 32 (suppl; abstr 9067)
Eggermont AM, Suciu S, Testori A et al (2012) Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol 30:3810–3818. doi:10.1200/JCO.2011.41.3799
Spatz A, Batist G, Eggermont AM (2010) The biology behind prognostic factors of cutaneous melanoma. Curr Opin Oncol 22:163–168. doi:10.1097/CCO.0b013e328337fe8f
Balch CM, Wilkerson JA, Murad TM, Soong SJ, Ingalls AL, Maddox WA (1980) The prognostic significance of ulceration of cutaneous melanoma. Cancer 45:3012–3017
Aviles-Izquierdo JA, Lazaro-Ochaita P (2012) Histological ulceration as a prognostic factor in cutaneous melanoma: a study of 423 cases in Spain. Clin Transl Oncol 14:237–240. doi:10.1007/s12094-012-0790-6
Eigentler TK, Buettner PG, Leiter U, Garbe C, Central Malignant Melanoma Registry of the German Dermatological S (2004) Impact of ulceration in stages I to III cutaneous melanoma as staged by the American Joint Committee on Cancer Staging System: an analysis of the German Central Malignant Melanoma Registry. J Clin Oncol. 22:4376–4383. doi:10.1200/JCO.2004.03.075
Balch CM, Soong SJ, Gershenwald JE et al (2001) Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 19:3622–3634
Kashani-Sabet M, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR 3rd (2002) Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J Clin Oncol 20:1826–1831
Jewell R, Elliott F, Laye J et al (2015) The clinicopathological and gene expression patterns associated with ulceration of primary melanoma. Pigment Cell Melanoma Res 28:94–104. doi:10.1111/pcmr.12315
Suciu S, Ives N, Eggermont AM, Kirkwood JM, Lorigan P, Markovic S, Garbe C, Wheatley K (2014) Predictive importance of ulceration on the efficacy of adjuvant interferon-a (IFN): an individual patient data (IPD) meta-analysis of 15 randomized trials in more than 7,500 melanoma patients (pts). J Clin Oncol 32 (suppl; abstr9067)
Grande Sarpa H, Reinke K, Shaikh L, Leong SP, Miller JR 3rd, Sagebiel RW, Kashani-Sabet M (2006) Prognostic significance of extent of ulceration in primary cutaneous melanoma. Am J Surg Pathol 30:1396–1400. doi:10.1097/01.pas.0000213262.61855.7d
Qian M, Ma MW, Fleming NH, Lackaye DJ, Hernando E, Osman I, Shao Y (2013) Clinicopathological characteristics at primary melanoma diagnosis as risk factors for brain metastasis. Melanoma Res 23:461–467. doi:10.1097/cmr.0000000000000015
Adams S, Gray RJ, Demaria S et al (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32:2959–2966. doi:10.1200/JCO.2013.55.0491
Loi S, Sirtaine N, Piette F et al (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31:860–867. doi:10.1200/JCO.2011.41.0902
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–128. doi:10.1126/science.aaa1348
Snyder A, Makarov V, Merghoub T et al (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371:2189–2199. doi:10.1056/NEJMoa1406498
Goldsmith LA, Fitzpatrick TB (2012) Fitzpatrick’s dermatology in general medicine. McGraw-Hill Medical, New York. Access Medicine. Web. September 28, 2014
Gallois A, Bhardwaj N (2013) Dendritic cell-targeted approaches to modulate immune dysfunction in the tumor microenvironment. Front Immunol 4:436. doi:10.3389/fimmu.2013.00436
Kashani-Sabet M (2010) Tumor progression by immune evasion in melanoma. Cancer 116:1623–1625. doi:10.1002/cncr.24909
Mandala M, Imberti GL, Piazzalunga D et al (2009) Clinical and histopathological risk factors to predict sentinel lymph node positivity, disease-free and overall survival in clinical stages I-II AJCC skin melanoma: outcome analysis from a single-institution prospectively collected database. Eur J Cancer 45:2537–2545. doi:10.1016/j.ejca.2009.05.034
Burton AL, Roach BA, Mays MP et al (2011) Prognostic significance of tumor infiltrating lymphocytes in melanoma. Am Surg 77:188–192
Bogunovic D, O’Neill DW, Belitskaya-Levy I et al (2009) Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci USA 106:20429–20434. doi:10.1073/pnas.0905139106
Harcharik S, Bernardo S, Moskalenko M et al (2014) Defining the role of CD2 in disease progression and overall survival among patients with completely resected stage-II to -III cutaneous melanoma. J Am Acad Dermatol 70:1036–1044. doi:10.1016/j.jaad.2014.01.914
Green JM, Karpitskiy V, Kimzey SL, Shaw AS (2000) Coordinate regulation of T cell activation by CD2 and CD28. J Immunol. 164:3591–3595
Tibaldi EV, Salgia R, Reinherz EL (2002) CD2 molecules redistribute to the uropod during T cell scanning: implications for cellular activation and immune surveillance. Proc Natl Acad Sci USA 99:7582–7587. doi:10.1073/pnas.112212699
Crawford K, Stark A, Kitchens B et al (2003) CD2 engagement induces dendritic cell activation: implications for immune surveillance and T-cell activation. Blood 102:1745–1752. doi:10.1182/blood-2002-07-2206
Mihm MC Jr, Clemente CG, Cascinelli N (1996) Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest 74:43–47
Capper D, Preusser M, Habel A et al (2011) Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol 122:11–19. doi:10.1007/s00401-011-0841-z
Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF (2012) Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 30:2678–2683. doi:10.1200/JCO.2011.37.8539
Safaee Ardekani G, Jafarnejad SM, Khosravi S, Martinka M, Ho V, Li G (2013) Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol 169:320–328. doi:10.1111/bjd.12351
Dong J, Phelps RG, Qiao R, Yao S, Benard O, Ronai Z, Aaronson SA (2003) BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer Res 63:3883–3885
van Rijn P, Rijmen F (2015) On the explaining-away phenomenon in multivariate latent variable models. Br J Math Stat Psychol 68:1–22. doi:10.1111/bmsp.12046
De Panfilis G, Campanini N, Santini M et al (2008) Phase- and stage-related proportions of T cells bearing the transcription factor FOXP3 infiltrate primary melanoma. J Invest Dermatol. 128:676–684. doi:10.1038/sj.jid.5701046
Balch CM, Gershenwald JE, Soong SJ et al (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199–6206. doi:10.1200/JCO.2009.23.4799
Fried L, Arbiser JL (2008) The reactive oxygen-driven tumor: relevance to melanoma. Pigment Cell Melanoma Res 21:117–122. doi:10.1111/j.1755-148X.2008.00451.x
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30:1073–1081. doi:10.1093/carcin/bgp127
Eggermont AM, Suciu S, Testori A et al (2012) Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer 48:218–225. doi:10.1016/j.ejca.2011.09.028
Baurain J, Stas M, Hammouch F et al (2009) Association of primary melanoma ulceration and clinical benefit of adjuvant vaccination with tumor-specific antigenic peptides. ASCO Meet Abstr 27:3022
Han D, Zager JS, Shyr Y et al (2013) Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol 31:4387–4393. doi:10.1200/JCO.2013.50.1114
Galon J, Angell HK, Bedognetti D, Marincola FM (2013) The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 39:11–26. doi:10.1016/j.immuni.2013.07.008
Acknowledgments
The study was funded by Dermatology Foundation (Career Development Award), American Association for Cancer Research (Landon Cancer Immunology Innovator Award), Herbert Irving Comprehensive Cancer Center at Columbia University, and Tisch Cancer Center at the Icahn School of Medicine at Mount Sinai.
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ellen H. de Moll and Yichun Fu have contributed equally to this article and should be considered co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Moll, E.H., Fu, Y., Qian, Y. et al. Immune biomarkers are more accurate in prediction of survival in ulcerated than in non-ulcerated primary melanomas. Cancer Immunol Immunother 64, 1193–1203 (2015). https://doi.org/10.1007/s00262-015-1726-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1726-0