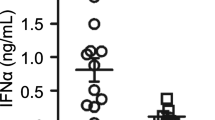
Abstract
Hepatocellular carcinoma (HCC) is a difficult to treat cancer characterized by poor tumor immunity with only one approved systemic drug, sorafenib. If novel combination treatments are to be developed with immunological agents, the effects of sorafenib on tumor immunity are important to understand. In this study, we investigate the impact of sorafenib on the CD4+CD25− effector T cells (Teff) and CD4+CD25+ regulatory T cells (Tregs) from patients with HCC. We isolated Teff and Treg from peripheral mononuclear cells of HCC patients to determine immune reactivity by thymidine incorporation, ELISA and flow cytometry. Teff cultured alone or with Treg were supplemented with different concentrations of sorafenib. The effects of sorafenib on Teff responses were dose-dependent. Pharmacologic doses of sorafenib decreased Teff activation by down regulating CD25 surface expression. In contrast, sub-pharmacologic concentrations of sorafenib resulted in Teff activation. These low doses of sorafenib in the Teff cultures led to a significant increase in Teff proliferation, IL2 secretion and up-regulation of CD25 expression on the cell surface. In addition, low doses of sorafenib in the suppression Teff/Treg cocultures restored Teff responses by eliminating Treg suppression. The loss of Treg suppressive function correlated with an increase in IL2 and IL6 secretion. Our findings show that sub-pharmacologic doses of sorafenib impact subsets of T cells differently, selectively increasing Teff activation while blocking Treg function. In conclusion, this study describes novel immune activating properties of low doses of sorafenib by promoting immune responsiveness in patients with HCC.






Similar content being viewed by others
References
El-Serag HB, Marrero JA, Rudolph L, Reddy KR (2008) Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 134:1752–1763
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics 2010. CA Cancer J Clin 60:277–300
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 1:25–34
Cao M, Cabrera R, Xu Y, Firpi R, Zhu H, Liu C, Nelson DR (2007) Hepatocellular carcinoma cell supernatants increase expansion and function of CD4(+)CD25(+) regulatory T cells. Lab Invest 87:582–590
Cabrera R, Ararat M, Cao M, Xu Y, Wasserfall C, Atkinson MA, Liu C, Nelson DR (2010) Hepatocellular carcinoma immunopathogenesis: clinical evidence for global T cell defects and an immunomodulatory role for soluble CD25 (sCD25). Dig Dis Sci 55:484–495
Li H, Zhao H, Yu J, Su Y, Cao S, An X, Ren X (2011) Increased prevalence of regulatory T cells in the lung cancer microenvironment: a role of thymic stromal lymphopoietin. Cancer Immunol Immunother 60:1587–1596
Feng X, Li B, Ye H, Long D (2011) Increased frequency of CD4(+)CD25 (high)FoxP3 (+) regulatory T Cells in patients with hepatocellular carcinoma. Arch Immunol Ther Exp (Warsz) 59:309–314
Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S (2007) FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 13:902–911
Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L (2009) Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer 125:1640–1648
Cabrera R, Ararat M, Eksioglu EA, Cao M, Xu Y, Wasserfall C, Atkinson MA, Liu C, Nelson DR (2010) Influence of serum and soluble CD25 (sCD25) on regulatory and effector T-cell function in hepatocellular carcinoma. Scand J Immunol 72:293–301
Houben R, Voigt H, Noelke C, Hofmeister V, Becker J, Schrama D (2009) MAPK-independent impairment of T-cell responses by the multikinase inhibitor sorafenib. Mol Cancer Ther 8:433–440
Zhao W, Gu YH, Song R, Qu BQ (2008) Xu Q Sorafenib inhibits activation of human peripheral blood T cells by targeting LCK phosphorylation. Leukemia 22:1226–1233
Toso C, Mentha G, Majno P (2011) Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. Am J Transplant 1:2031–2035
Villanueva A, Llovet JM (2011) Targeted therapies for hepatocellular carcinoma. Gastroenterology 140:1410–1426
Blanchet B, Billemont B, Cramard J, Benichou AS, Chhun S, Harcouet L, Ropert S, Dauphin A, Goldwasser F, Tod M (2009) Validation of an HPLC-UV method for sorafenib determination in human plasma and application to cancer patients in routine clinical practice. J Pharm Biomed Anal 49:1109–1114
Lavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C (2011) On behalf of the SOFIA (Sorafenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology 54:2055–2063
Lencioni R, Marrero J, Venook A, Ye SL, Kudo M (2010) Design and rationale for the non-interventional global investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafenib (GIDEON) study. Int J Clin Pract 64:1034–1041
Heinz WJ, Kahle K, Helle-Beyersdorf A, Schirmer D, Lenker U, Keller D, Langmann P, Klinker H (2011) High-performance liquid chromatographic method for the determination of sorafenib in human serum and peritoneal fluid. Cancer Chemother Pharmacol 68:239–245
Abou-Alfa GK, Amadori D, Santoro A, Figer A, De Greve J, Lathia C, Voliotis D, Anderson S, Moscovici M, Ricci S (2011) Safety and efficacy of sorafenib in patients with hepatocellular carcinoma (HCC) and Child–Pugh A versus B cirrhosis. Gastrointest Cancer Res 42:40–44
Arteaga C, Baselga J (2004) Tyrosyne kinase inhibitors: why does the current process of clinical development not apply to them? Cancer Cell 5:525–531
Turk JL, Parker D (1982) Effect of cyclophosphamide on immunological control mechanisms. Immunol Rev 65:99–113
Cao M, Xu Y, Youn JI, Cabrera R, Zhang X, Gabrilovich D, Nelson DR, Liu C (2011) Kinase inhibitor sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab Invest 9:598–608
Krusch M, Salih J, Schlicke M, Baessler T, Kampa KM, Mayer F, Salih HR (2009) The kinase inhibitors sunitinib and sorafenib differentially affect NK cell antitumor reactivity in vitro. J Immunol 183:8286–8294
Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, Singh-Jasuja H, Brossart P (2008) Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 111:5610–5620
Busse A, Asemissen AM, Nonnenmacher A, Braun F, Ochsenreither S, Stather D, Fusi A, Schmittel A, Miller K, Thiel E, Keilholz U (2011) Immunomodulatory effects of sorafenib on peripheral immune effector cells in metastatic renal cell carcinoma. Eur J Cancer 47:690–696
Brusko TM, Wasserfall CH, Hulme MA, Cabrera R, Schatz D, Atkinson MA (2009) Influence of membrane CD25 stability on T lymphocyte activity: implications for immunoregulation. PLoS One 4:e7980
Shevach EM (2009) Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30:636–645
Wang G, Khattar M, Guo Z, Miyahara Y, Linkes SP, Sun Z, He X, Stepkowski SM, Chen W (2010) IL-2-deprivation and TGF-beta are two non-redundant suppressor mechanisms of CD4+CD25+ regulatory T cell which jointly restrain CD4+CD25− cell activation. Immunol Lett 132:61–68
Von Boehmer H (2005) Mechanisms of suppression by suppressor T cells. Nat Immunol 6:338–344
Feinerman O, Jentsch G, Tkach KE, Coward JW, Hathorn MM, Sneddon MW, Emonet T, Smith KA, Altan-Bonnet G (2010) Single-cell quantification of IL-2 response by effector and regulatory T cells reveals critical plasticity in immune response. Mol Syst Biol 6:437
Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA (2004) Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol 172:5287–5296
Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, Bensinger SJ, Hancock WW, Turka LA (2006) PTEN inhibits IL-2 receptor-mediated expansion of CD4+CD25+ Tregs. Clin Invest 116:2521–2531
Acknowledgments
The authors want to thank Neal Benson and Lynn Combee for expert technical assistance with the flow cytometer experiments. Research support for this study provided in part by National Institutes of Health/National Center for Research Resources Award UL1 RR029890 and National Institutes of Health/National Cancer Institute award K24CA139570.
Conflict of interest
Dr. Cabrera is a speaker, consultant, and has research grants from Bayer. Dr. Nelson is a consultant and has research grants from Bayer and Human Genome Science.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

262_2012_1380_MOESM1_ESM.jpg
Supplemental Figure 1 Low Doses of sorafenib does not impact cell function in controls. CD4+ T effector cells from normal healthy controls (NHC) and patients with well compensated cirrhosis but no cancer (DC) (n=6) were stimulated with PHA without sorafenib and with low doses of sorafenib (0.1uM and 1uM). No significant differences were observed in the normal healthy controls and disease controls when their PBMCs were stimulated with and without sorafenib. Supplementary material 1 (JPEG 158 kb)
Rights and permissions
About this article
Cite this article
Cabrera, R., Ararat, M., Xu, Y. et al. Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother 62, 737–746 (2013). https://doi.org/10.1007/s00262-012-1380-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1380-8