Abstract
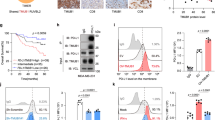
Imatinib (IM) has been described to modulate the function of dendritic cells and T lymphocytes and to affect the expression of antigen in CML cells. In our study, we investigated the effect of the tyrosine kinase inhibitors IM and nilotinib (NI) on antigen presentation and processing by analyzing the proteasomal activity in CML cell lines and patient samples. We used a biotinylated active site-directed probe, which covalently binds to the proteasomally active beta-subunits in an activity-dependent fashion. Additionally, we analyzed the cleavage and processing of HLA-A3/11- and HLA-B8-binding peptides derived from BCR-ABL by IM- or NI-treated isolated 20S immunoproteasomes using mass spectrometry. We found that IM treatment leads to a reduction in MHC-class I expression which is in line with the inhibition of proteasomal activity. This process is independent of BCR-ABL or apoptosis induction. In vitro digestion experiments using purified proteasomes showed that generation of epitope-precursor peptides was significantly altered in the presence of NI and IM. Treatment of the immunoproteasome with these compounds resulted in an almost complete reduction in the generation of long precursor peptides for the HLA-A3/A11 and −B8 epitopes while processing of the short peptide sequences increased. Treatment of isolated 20S proteasomes with serine-/threonine- and tyrosine-specific phosphatases induced a significant downregulation of the proteasomal activity further indicating that phosphorylation of the proteasome regulates its function and antigen processing. Our results demonstrate that IM and NI can affect the immunogenicity of malignant cells by modulating proteasomal degradation and the repertoire of processed T cell epitopes.






Similar content being viewed by others
References
Lugo TG, Pendergast AM, Muller AJ, Witte ON (1990) Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 247:1079–1082
Heisterkamp N, Jenster G, ten Hoeve J, Zovich D, Pattengale PK, Groffen J (1990) Acute leukaemia in bcr/abl transgenic mice. Nature 344:251–253. doi:10.1038/344251a0
Hochhaus A, O’Brien SG, Guilhot F et al (2009) Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 23:1054–1061. doi:10.1038/leu.2009.38
Kantarjian HM, O’Brien S, Cortes J et al (2003) Imatinib mesylate therapy improves survival in patients with newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in the chronic phase: comparison with historic data. Cancer 98:2636–2642. doi:10.1002/cncr.11831
Roy L, Guilhot J, Krahnke T et al (2006) Survival advantage from imatinib compared with the combination interferon-alpha plus cytarabine in chronic-phase chronic myelogenous leukemia: historical comparison between two phase 3 trials. Blood 108:1478–1484. doi:10.1182/blood-2006-02-001495
Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J (2000) Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289:1938–1942
O’Brien SG, Guilhot F, Larson RA et al (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348:994–1004. doi:10.1056/NEJMoa022457
Snead JL, O’Hare T, Eide CA, Deininger MW (2008) New strategies for the first-line treatment of chronic myeloid leukemia: can resistance be avoided? Clin Lymphoma Myeloma 8(Suppl 3):S107–S117. doi:10.3816/CLM.2008.s.006
Talpaz M, Shah NP, Kantarjian H et al (2006) Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 354:2531–2541. doi:10.1056/NEJMoa055229
Heine A, Held SA, Bringmann A, Holderried TA, Brossart P (2011) Immunomodulatory effects of anti-angiogenic drugs. Leukemia 25:899–905. doi:10.1038/leu.2011.24
Appel S, Boehmler AM, Grunebach F et al (2004) Imatinib mesylate affects the development and function of dendritic cells generated from CD34+ peripheral blood progenitor cells. Blood 103:538–544. doi:10.1182/blood-2003-03-0975
Balabanov S, Appel S, Kanz L, Brossart P, Brummendorf TH (2005) Effect of tyrosine kinase inhibition using imatinib on normal lymphohematopoietic cells. Ann N Y Acad Sci 1044:168–177. doi:10.1196/annals.1349.022
Appel S, Rupf A, Weck MM, Schoor O, Brummendorf TH, Weinschenk T, Grunebach F, Brossart P (2005) Effects of imatinib on monocyte-derived dendritic cells are mediated by inhibition of nuclear factor-kappaB and Akt signaling pathways. Clin Cancer Res 11:1928–1940. doi:10.1158/1078-0432.CCR-04-1713
Brauer KM, Werth D, von Schwarzenberg K, Bringmann A, Kanz L, Grunebach F, Brossart P (2007) BCR-ABL activity is critical for the immunogenicity of chronic myelogenous leukemia cells. Cancer Res 67:5489–5497. doi:10.1158/0008-5472.CAN-07-0302
Salih J, Hilpert J, Placke T, Grunebach F, Steinle A, Salih HR, Krusch M (2010) The BCR/ABL-inhibitors imatinib, nilotinib and dasatinib differentially affect NK cell reactivity. Int J Cancer 127:2119–2128. doi:10.1002/ijc.25233
Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC, Dunbar CE, Wiestner A (2005) Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 105:2473–2479. doi:10.1182/blood-2004-07-2527
Dietz AB, Souan L, Knutson GJ, Bulur PA, Litzow MR, Vuk-Pavlovic S (2004) Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood 104:1094–1099. doi:10.1182/blood-2003-12-4266
Reichardt VL, Brossart P (2005) Current status of vaccination therapy for leukemias. Curr Hematol Rep 4:73–76
Takahashi T, Tanaka Y, Nieda M, Azuma T, Chiba S, Juji T, Shibata Y, Hirai H (2003) Dendritic cell vaccination for patients with chronic myelogenous leukemia. Leuk Res 27:795–802
Weisberg E, Griffin JD (2000) Mechanism of resistance to the ABL tyrosine kinase inhibitor STI571 in BCR/ABL-transformed hematopoietic cell lines. Blood 95:3498–3505
Appel S, Mirakaj V, Bringmann A, Weck MM, Grunebach F, Brossart P (2005) PPAR-gamma agonists inhibit toll-like receptor-mediated activation of dendritic cells via the MAP kinase and NF-kappaB pathways. Blood 106:3888–3894. doi:10.1182/blood-2004-12-4709
Appel S, Bringmann A, Grunebach F, Weck MM, Bauer J, Brossart P (2006) Epithelial-specific transcription factor ESE-3 is involved in the development of monocyte-derived DCs. Blood 107:3265–3270. doi:10.1182/blood-2005-06-2480
le Coutre P, Kreuzer KA, Pursche S et al (2004) Pharmacokinetics and cellular uptake of imatinib and its main metabolite CGP74588. Cancer Chemother Pharmacol 53:313–323. doi:10.1007/s00280-003-0741-6
Kantarjian H, Giles F, Wunderle L et al (2006) Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 354:2542–2551. doi:10.1056/NEJMoa055104
Faivre S, Delbaldo C, Vera K et al (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35. doi:10.1200/JCO.2005.02.2194
Strumberg D, Richly H, Hilger RA et al (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol 23:965–972. doi:10.1200/JCO.2005.06.124
Berkers CR, Verdoes M, Lichtman E, Fiebiger E, Kessler BM, Anderson KC, Ploegh HL, Ovaa H, Galardy PJ (2005) Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat Methods 2:357–362. doi:10.1038/nmeth759
Bogyo M, McMaster JS, Gaczynska M, Tortorella D, Goldberg AL, Ploegh H (1997) Covalent modification of the active site threonine of proteasomal beta subunits and the Escherichia coli homolog HslV by a new class of inhibitors. Proc Natl Acad Sci USA 94:6629–6634
Kessler BM, Tortorella D, Altun M, Kisselev AF, Fiebiger E, Hekking BG, Ploegh HL, Overkleeft HS (2001) Extended peptide-based inhibitors efficiently target the proteasome and reveal overlapping specificities of the catalytic beta-subunits. Chem Biol 8:913–929
Nencioni A, Schwarzenberg K, Brauer KM, Schmidt SM, Ballestrero A, Grunebach F, Brossart P (2006) Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood 108:551–558. doi:10.1182/blood-2005-08-3494
Nencioni A, Garuti A, Schwarzenberg K et al (2006) Proteasome inhibitor-induced apoptosis in human monocyte-derived dendritic cells. Eur J Immunol 36:681–689. doi:10.1002/eji.200535298
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139:271–279
Tenzer S, Stoltze L, Schonfisch B, Dengjel J, Muller M, Stevanovic S, Rammensee HG, Schild H (2004) Quantitative analysis of prion-protein degradation by constitutive and immuno-20S proteasomes indicates differences correlated with disease susceptibility. J Immunol 172:1083–1091
Silva JC, Denny R, Dorschel CA et al (2005) Quantitative proteomic analysis by accurate mass retention time pairs. Anal Chem 77:2187–2200. doi:10.1021/ac048455k
Silva JC, Gorenstein MV, Li GZ, Vissers JP, Geromanos SJ (2006) Absolute quantification of proteins by LCMSE: a virtue of parallel MS acquisition. Mol Cell Proteomics MCP 5:144–156. doi:10.1074/mcp.M500230-MCP200
Bocchia M, Wentworth PA, Southwood S, Sidney J, McGraw K, Scheinberg DA, Sette A (1995) Specific binding of leukemia oncogene fusion protein peptides to HLA class I molecules. Blood 85:2680–2684
Clark RE, Dodi IA, Hill SC et al (2001) Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood 98:2887–2893
Posthuma EF, van Bergen CA, Kester MG et al (2004) Proteosomal degradation of BCR/ABL protein can generate an HLA-A*0301-restricted peptide, but high-avidity T cells recognizing this leukemia-specific antigen were not demonstrated. Haematologica 89:1062–1071
Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, Singh-Jasuja H, Brossart P (2008) Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 111:5610–5620. doi:10.1182/blood-2007-02-075945
Burchert A, Wolfl S, Schmidt M et al (2003) Interferon-alpha, but not the ABL-kinase inhibitor imatinib (STI571), induces expression of myeloblastin and a specific T-cell response in chronic myeloid leukemia. Blood 101:259–264. doi:10.1182/blood-2002-02-0659
Borg C, Terme M, Taieb J et al (2004) Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Investig 114:379–388. doi:10.1172/JCI21102
Crawford LJ, Windrum P, Magill L, Melo JV, McCallum L, McMullin MF, Ovaa H, Walker B, Irvine AE (2009) Proteasome proteolytic profile is linked to Bcr-Abl expression. Exp Hematol 37:357–366. doi:10.1016/j.exphem.2008.11.004
Bocchia M, Korontsvit T, Xu Q, Mackinnon S, Yang SY, Sette A, Scheinberg DA (1996) Specific human cellular immunity to bcr-abl oncogene-derived peptides. Blood 87:3587–3592
Yotnda P, Firat H, Garcia-Pons F, Garcia Z, Gourru G, Vernant JP, Lemonnier FA, Leblond V, Langlade-Demoyen P (1998) Cytotoxic T cell response against the chimeric p210 BCR-ABL protein in patients with chronic myelogenous leukemia. J Clin Investig 101:2290–2296. doi:10.1172/JCI488
Lu H, Zong C, Wang Y et al (2008) Revealing the dynamics of the 20 S proteasome phosphoproteome: a combined CID and electron transfer dissociation approach. Mol Cell Proteomics MCP 7:2073–2089. doi:10.1074/mcp.M800064-MCP200
Acknowledgments
We thank Solveig Daecke and Anika Beckers for their excellent technical assistance. This work was supported by grants from Deutsche Forschungsgemeinschaft (DFG) (PB), Deutsche Krebshilfe (PB), BONFOR Forschungsförderung (AH) and SFB 704 (PB). Parts of this work were presented on the annual meeting of the American Society of Hematology (ASH) as a poster under the same title.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. A. E. Held and K. M. Duchardt contributed equally to this work.
Rights and permissions
About this article
Cite this article
Held, S.A.E., Duchardt, K.M., Tenzer, S. et al. Imatinib mesylate and nilotinib affect MHC-class I presentation by modulating the proteasomal processing of antigenic peptides. Cancer Immunol Immunother 62, 715–726 (2013). https://doi.org/10.1007/s00262-012-1373-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1373-7