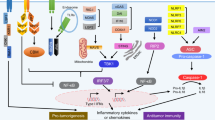
Abstract
A subset of patients with a variety of cancers shows evidence of a natural adaptive immune response against their tumor, as evidenced by spontaneous T-cell infiltration, circulating anti-tumor T cells, or antibody responses. Evidence has indicated that such natural immune responses have positive prognostic import in early stage disease and may be predictive of clinical response to immunotherapeutics in advanced disease. However, these observations raise a new critical fundamental question—what innate immune signals might be generated in the context of non-pathogen-induced cancers that drive productive antigen presentation toward induction of an adaptive immune response? Gene expression profiling in melanoma revealed that tumors having high expression of T-cell markers also show evidence of a type I IFN transcriptional signature. Mechanistic experiments in mice have revealed that a spontaneous CD8+ T-cell response against transplantable tumors depends on host type I IFN signaling, through a mechanism dependent upon CD8α+ dendritic cells (DCs). The requirement for type I IFN production by host DCs has suggested a subset of innate immune sensing receptors and signaling pathways that might be involved with initiating this process. Elucidating further these innate immune mechanisms should provide new insights into cancer immunotherapy.

Similar content being viewed by others
References
Galon J, Costes A, Sanchez-Cabo F et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Mlecnik B, Tosolini M, Kirilovsky A et al (2011) Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 29:610–618
Mahmoud SM, Paish EC, Powe DG et al (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29:1949–1955
Zhang L, Conejo-Garcia JR, Katsaros D et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Elder DE, Van Belle P, Elenitsas R et al (1996) Neoplastic progression and prognosis in melanoma. Semin Cutan Med Surg 15:336–348
Gajewski TF, Zha Y, Thurner B, Schuler G (2009) Association of gene expression profile in melanoma and survival to a dendritic cell-based vaccine. J Clin Oncol 27:9002
Harlin H, Meng Y, Peterson AC et al (2009) Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 69:3077–3085
Gajewski TF, Louahed J, Brichard VG (2010) Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J 16:399–403
Sullivan RJ, Hoshida Y, Brunet J et al (2009) A single center experience with high-dose IL-2 treatment for patients with advanced melanoma and pilot investigation of a novel gene expression signature as a predictor of response. J Clin Oncol 27:15S (Abstract 9003)
Hamid O, Schmidt H, Nissan A et al (2011) A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 9:204
Ji RR, Chasalow SD, Wang L et al (2011) An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother
Harlin H, Kuna TV, Peterson AC et al (2006) Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother 55:1185–1197
Appay V, Jandus C, Voelter V et al (2006) New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol 177:1670–1678
Mortarini R, Piris A, Maurichi A et al (2003) Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res 63:2535–2545
Valmori D, Dutoit V, Rubio-Godoy V et al (2001) Frequent cytolytic T-cell responses to peptide MAGE-A10(254–262) in melanoma. Cancer Res 61:509–512
Peterson AC, Harlin H, Gajewski TF (2003) Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J Clin Oncol 21:2342–2348
Jager E, Chen YT, Drijfhout JW et al (1998) Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med 187:265–270
Darnell RB, Posner JB (2006) Paraneoplastic syndromes affecting the nervous system. Semin Oncol 33:270–298
Wang X, Yu J, Sreekumar A et al (2005) Autoantibody signatures in prostate cancer. N Engl J Med 353:1224–1235
Gajewski TF (2007) Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res 13:5256–5261
Blank C, Brown I, Peterson AC et al (2004) PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res 64:1140–1145
Brown IE, Blank C, Kline J et al (2006) Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol 177:4521–4529
Kline J, Brown IE, Zha YY et al (2008) Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res 14:3156–3167
Uyttenhove C, Pilotte L, Theate I et al (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9:1269–1274
Fuertes MB, Kacha AK, Kline J et al (2011) Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med
Sancho D, Joffre OP, Keller AM et al (2009) Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 458:899–903
Diamond MS, Kinder M, Matsushita H et al (2011) Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 208:1989–2003
Hildner K, Edelson BT, Purtha WE et al (2008) Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322:1097–1100
Barton GM, Medzhitov R (2002) Toll-like receptors and their ligands. Curr Top Microbiol Immunol 270:81–92
Martinon F, Mayor A, Tschopp J (2009) The inflammasomes: guardians of the body. Annu Rev Immunol 27:229–265
Sancho D, Mourao-Sa D, Joffre OP et al (2008) Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest 118:2098–2110
Ishikawa H, Barber GN (2008) STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674–678
McCartney S, Vermi W, Gilfillan S et al (2009) Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med 206:2967–2976
Barber GN (2011) Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol 23:10–20
Lister MF, Sharkey J, Sawatzky DA et al (2007) The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 4:5
Muller T, Vieira RP, Grimm M et al (2011) A potential role for P2X7R in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol 44:456–464
Apetoh L, Ghiringhelli F, Tesniere A et al (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059
Ghiringhelli F, Apetoh L, Tesniere A et al (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 15:1170–1178
Niewold TB, Hua J, Lehman TJ et al (2007) High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun 8:492–502
Salloum R, Franek BS, Kariuki SN et al (2010) Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon-alpha activity in lupus patients. Arthr Rheum 62:553–561
Acknowledgment
This work was supported by P01 CA97296 from the National Cancer Institute, USA.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is a Focussed Research Review based on a presentation given at the Eleventh International Conference on Progress in Vaccination against Cancer (PIVAC 11), held in Copenhagen, Denmark, 10th–13th October 2011. It is part of a CII series of Focussed Research Reviews and meeting report.
Rights and permissions
About this article
Cite this article
Gajewski, T.F., Fuertes, M.B. & Woo, SR. Innate immune sensing of cancer: clues from an identified role for type I IFNs. Cancer Immunol Immunother 61, 1343–1347 (2012). https://doi.org/10.1007/s00262-012-1305-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1305-6