Abstract
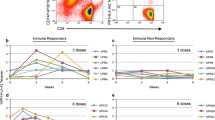
Survivin, a member of the inhibitor of apoptosis protein family, is expressed in many malignant tumors including urothelial cancer but is hardly detectable in normal, differentiated adult tissues. Previously we reported CD8-positive cytotoxic T-lymphocytes (CTLs) were successfully induced by stimulation with survivin-2B80-88 peptide in vitro. We started a phase I clinical study of survivin-2B80-88 peptide vaccination for advanced urothelial cancer patients to assess the safety and efficacy of this vaccination. Nine patients were received vaccination and were evaluated for immunological evaluation, adverse events, and clinical responses. A total of 46 vaccinations were carried out. There was no severe adverse event. HLA-A24/survivin-2B80-88 peptide tetramer analysis revealed a significant increase in the peptide-specific CTL frequency after the vaccination in five patients. Slight reduction of the tumor volume was observed in one patient. Survivin-2B80-88 peptide-based vaccination is safe and should be further considered for potential immune and clinical efficacy in urothelial cancer patients.



Similar content being viewed by others
Abbreviations
- CR:
-
Complete response
- CT:
-
Computed tomography
- CTL:
-
Cytotoxic T-lymphocyte
- DTH:
-
Delayed-type hypersensitivity
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigen
- HSP:
-
Heat shock protein
- IFA:
-
Incomplete Freund’s adjuvant
- IFN:
-
Interferon
- NC:
-
No change
- PBMC:
-
Peripheral blood mononuclear cell
- PD:
-
Progressive disease
- PR:
-
Partial response
References
Rosenberg SA (1999) A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity 10:281–287
Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917–921
Kawasaki H, Altieri DC, Lu CD et al (1998) Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res 58:5071–5074
Tamm I, Wang Y, Sausville E et al (1998) IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 58:5315–5320
Hirohashi Y, Torigoe T, Maeda A et al (2002) An HLA-A24-restricted cytotoxic T lymphocyte epitope of a tumor-associated protein, survivin. Clin Cancer Res 8:1731–1739
Idenoue S, Hirohashi Y, Torigoe T et al (2005) A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res 11:1474–1482
Tsuruma T, Hata F, Torigoe T et al (2004) Phase I clinical study of anti-apoptosis protein, survivinderived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med 2:19–29
Tsuruma T, Iwayama Y, Ohmura T et al (2008) Clinical and immunological evaluation of anti-apoptosis protein, survivin-derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med 6:24
Kitamura H, Torigoe T, Honma I et al (2006) Expression and antigenicity of survivin, an inhibitor of apoptosis family member, in bladder cancer: implications for specific immunotherapy. Urology 67:955–959
Koh YT, Higgins SA, Weber JS et al (2006) Immunological consequences of using three different clinical/laboratory techniques of emulsifying peptide-based vaccines in incomplete Freund’s adjuvant. J Trans Med 4:42
Altman JD, Moss PA, Goulder PJ et al (1996) Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94
Sato Y, Sahara H, Tsukahara T et al (2002) Improved generation of HLA class/peptide tetramers. J Immunol Methods 20:177–184
Sato Y, Nabeta Y, Tsukahara T et al (2002) Detection and induction of CTLs specific for SYT-SSX-derived peptides in HLA-A24(+) patients with synovial sarcoma. J Immunol 169:1611–1618
Date Y, Kimura A, Kato H et al (1998) DNA typing of the HLA-A gene: population study and identification of four new alleles in Japanese. Tissue Antigens 47:93–101
Nestle FO, Alijagic S, Gilliet M et al (1998) Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 4:328–332
Andersen MH, Pedersen LO, Capeller B et al (2001) Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res 61:5964–5968
Ojima T, Iwahashi M, Nakamura M et al (2006) The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen. Int J Oncol 28:947–953
Svane IM, Nikolajsen K, Walter MR et al (2006) Characterization of monocyte-derived dendritic cells maturated with IFN-alpha. Scand J Immunol 63:217–222
Kurotaki T, Tamura Y, Ueda G et al (2007) Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J Immunol 179:1803–1813
Kumagai Y, Takeuchi O, Akira S (2008) TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev 60:795–804
Acknowledgments
Kumiko Shimozawa and Emiri Nakazawa provided technical assistance. This study was supported in part by a grant-in-aid for Clinical Cancer Research from the Ministry of Health, Labor and Welfare and a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 17390441 to T. Tsukamoto), and research grants from the Stiftelsen Japanese-Swedish Research Foundation, and Gotaro Sugawara Research Fund for Urological Diseases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honma, I., Kitamura, H., Torigoe, T. et al. Phase I clinical study of anti-apoptosis protein survivin-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother 58, 1801–1807 (2009). https://doi.org/10.1007/s00262-009-0691-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0691-x