Abstract
Introduction
Vγ9Vδ2 T lymphocytes are reported to participate in the anti-tumor immune surveillance in human. They are known to recognize phosphoantigens and molecules expressed on cells undergoing neoplasic transformation. In this study, we investigated phenotype and anti-tumor cytotoxicity of ex vivo expanded Vγ9Vδ2 T cells in view of adoptive immunotherapy.
Materials and Methods
Experiments were performed with peripheral blood samples from eleven patients [six colorectal carcinoma (CRC), four hepatocellular carcinoma (HCC), one sarcoma] and sixteen healthy donors.
Results/Discussion
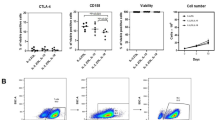
Ex vivo expansion of Vγ9Vδ2 T cells could be achieved by a single dose of phosphoantigen, either bromohydrin pyrophosphate or zoledronate, and supported by exogenous IL-2. After 2 weeks, expanded Vγ9Vδ2 T lymphocytes acquired the effector memory phenotype CD45RA−CD45ROhighCD27−. They expressed NKG2D and CD161 and the proinflammatory CXCR3 and CCR5 chemokine receptors. Vγ9Vδ2 T cells displayed a strong lytic activity toward a broad panel of tumor cell lines or primary cultures. Interestingly, HCC and CRC primary cells could be lysed by autologous Vγ9Vδ2 T cells whereas autologous normal cells were not sensitive to the lysis. mAbs blocking assays demonstrated that TCR was the most important receptor involved in the lysis of tumor cells. However, NKG2D receptor could deliver a costimulatory signal enhancing the lysis of HCC and CRC tumors expressing MICA/B. Treatment of tumor cells by the mevalonate pathway inhibitor, zoledronate, enhanced the killing of both HCC and CRC. Expansion index of Vγ9Vδ2 T cells was in similar levels in healthy donors or in cancer patients and total expansion was suitable for adoptive immunotherapy.
Conclusion
These results provide a rationale for the clinical evaluation of Vγ9Vδ2 T lymphocytes in HCC and CRC.







Similar content being viewed by others
Abbreviations
- IPP:
-
Isopentenyl pyrophosphate
- BrHPP:
-
Bromohydrin pyrophosphate
- HCC:
-
Hepatocellular carcinoma
- CRC:
-
Colorectal carcinoma
- RCC:
-
Renal cell carcinoma
- HSP:
-
Heat shock protein
- MICA/B:
-
MHC class I chain-related
- ULBP:
-
UL16-binding protein
References
Aldemir H, Prod’homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, Bihl F, Braud VM (2005) Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol 175:7791–7795
Barkholt L, Danielsson R, Calissendorff B, Svensson L, Malihi R, Remberger M, Uzunel M, Thorne A, Ringden O (2004) Indium-111-labelled donor-lymphocyte infusion by way of hepatic artery and radio-frequency ablation against liver metastases of renal and colon carcinoma after allogeneic hematopoietic stem-cell transplantation. Transplantation 78:697–703
Battistini L, Caccamo N, Borsellino G, Meraviglia S, Angelini DF, Dieli F, Cencioni MT, Salerno A (2005) Homing and memory patterns of human gammadelta T cells in physiopathological situations. Microbes Infect 7:510–517
Blattman JN, Greenberg PD (2004) Cancer immunotherapy: a treatment for the masses. Science 305:200–205
Bonneville M, Fournie JJ (2005) Sensing cell stress and transformation through Vgamma9Vdelta2 T cell-mediated recognition of the isoprenoid pathway metabolites. Microbes Infect 7:503–509
Bonneville M, Scotet E (2006) Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol 18:539–546
Choudhary A, Davodeau F, Moreau A, Peyrat MA, Bonneville M, Jotereau F (1995) Selective lysis of autologous tumor cells by recurrent gamma delta tumor-infiltrating lymphocytes from renal carcinoma. J Immunol 154:3932–3940
Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, Bonneville M, Jotereau F (2005) V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol 175:5481–5488
Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A (2003) Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med 198:391–397
Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA (2005) Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 23:2346–2357
Exley M, Porcelli S, Furman M, Garcia J, Balk S (1998) CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant V alpha 24 J alpha Q T cell receptor alpha chains. J Exp Med 188:867–876
Favier B, Espinosa E, Tabiasco J, Dos Santos C, Bonneville M, Valitutti S, Fournie JJ (2003) Uncoupling between immunological synapse formation and functional outcome in human gamma delta T lymphocytes. J Immunol 171:5027–5033
Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR (2002) Human gammadelta T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol 23:14–18
Girardi M (2006) Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol 126:25–31
Glaise D, Ilyin GP, Loyer P, Cariou S, Bilodeau M, Lucas J, Puisieux A, Ozturk M, Guguen-Guillouzo C (1998) Cell cycle gene regulation in reversibly differentiated new human hepatoma cell lines. Cell Growth Differ 9:165–176
Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G (2003) Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 197:163–168
Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T (2001) Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol 2:255–260
Hayday A, Tigelaar R (2003) Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol 3:233–242
Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H (2007) Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother 56:469–476
Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23:225–274
Morita CT, Jin C, Sarikonda G, Wang H (2007) Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 215:59–76
Pennington DJ, Silva-Santos B, Hayday AC (2005) Gammadelta T cell development—having the strength to get there. Curr Opin Immunol 17:108–115
Poggi A, Zocchi MR, Costa P, Ferrero E, Borsellino G, Placido R, Galgani S, Salvetti M, Gasperini C, Ristori G, Brosnan CF, Battistini L (1999) IL-12-mediated NKRP1A up-regulation and consequent enhancement of endothelial transmigration of V delta 2+ TCR gamma delta+ T lymphocytes from healthy donors and multiple sclerosis patients. J Immunol 162:4349–4354
Raulet DH (2003) Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 3:781–790
Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL (2005) Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol 175:7796–7799
Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H, Maekawa T (2005) Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer 116:94–99
Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Esteve JP, Lopez F, Perret B, Collet X, Bonneville M, Champagne E (2005) Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 22:71–80
Takayama T, Makuuchi M, Sekine T, Terui S, Shiraiwa H, Kosuge T, Yamazaki S, Hasegawa H, Suzuki K, Yamagata M, et al (1991) Distribution and therapeutic effect of intraarterially transferred tumor-infiltrating lymphocytes in hepatic malignancies. A preliminary report. Cancer 68:2391–2396
Thomas ML, Samant UC, Deshpande RK, Chiplunkar SV (2000) gammadelta T cells lyse autologous and allogenic oesophageal tumours: involvement of heat-shock proteins in the tumour cell lysis. Cancer Immunol Immunother 48:653–659
Viey E, Fromont G, Escudier B, Morel Y, Da Rocha S, Chouaib S, Caignard A (2005) Phosphostim-activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J Immunol 174:1338–1347
Vivier E, Tomasello E, Paul P (2002) Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr Opin Immunol 14:306–311
Wadia P, Atre N, Pradhan T, Mistry R, Chiplunkar S (2005) Heat shock protein induced TCR gammadelta gene rearrangements in patients with oral cancer. Oral Oncol 41:175–182
Wang MH, Chen YQ, Gercken J, Ernst M, Bohle A, Flad HD, Ulmer AJ (1993) Specific activation of human peripheral blood gamma/delta + lymphocytes by sonicated antigens of Mycobacterium tuberculosis: role in vitro in killing human bladder carcinoma cell lines. Scand J Immunol 38:239–246
Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP (2003) Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 102:200–206
Zhang H, Wang W, Zhang S, Huang W (2005) Comparison of the anti-tumor effects of various whole-body hyperthermia protocols: correlation with HSP 70 expression and composition of splenic lymphocytes. Immunol Invest 34:245–258
Zheng BJ, Ng SP, Chua DT, Sham JS, Kwong DL, Lam CK, Ng MH (2002) Peripheral gamma delta T-cell deficit in nasopharyngeal carcinoma. Int J Cancer 99:213–217
Zocchi MR, Ferrarini M, Rugarli C (1990) Selective lysis of the autologous tumor by delta TCS1+ gamma/delta+ tumor-infiltrating lymphocytes from human lung carcinomas. Eur J Immunol 20:2685–2689
Acknowledgments
This work was supported by grants from the Comité Grand Ouest de la Ligue Contre le Cancer, the GIS de Thérapie Cellulaire de Rennes and the Institut National du Cancer (PL074). We thank E. Gougeon for technical assistance, D. Glaise and J. Le Seyec (INSERM U522, Rennes) for providing HCC cell lines and expert assistance in culture of normal hepatocytes, J-J. Fournié (INSERM U563, Toulouse) and Innate Pharma (Marseille) for providing BrHPP, V. Quillien (CRLCC, Rennes) for providing GB2 and GB3 glioblastoma primary cultures, and N. Rioux for anatomopathological examinations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bouet-Toussaint, F., Cabillic, F., Toutirais, O. et al. Vγ9Vδ2 T cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas. Cancer Immunol Immunother 57, 531–539 (2008). https://doi.org/10.1007/s00262-007-0391-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-007-0391-3