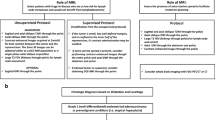
Abstract
Background
Prostate multiparametric magnetic resonance imaging (mpMRI) can identify lesions within the prostate with characteristics identified in Prostate Imaging Reporting and Data System (PI-RADS) v2.1 associated with clinically significant prostate cancer (csPCa) or Gleason grade group (GGG) ≥ 2 at biopsy.
Objective
To assess concordance (PI-RADS 5 lesions with csPCa) of PI-RADS v2/2.1 with targeted, fusion biopsy results and to examine causes of discordance (PI-RADS 5 lesions without csPCa) with aim to provide a structured approach to resolving discordances and develop quality improvement (QI) protocols.
Methods
A retrospective study of 392 patients who underwent mpMRI at 3 Tesla followed by fusion biopsy. PI-RADS v2/2.1 scores were assigned to lesions identified on mpMRI and compared to biopsy results expressed as GGG. Positive predictive value (PPV) of PI-RADS v2/2.1 was calculated for all prostate cancer and csPCa. Discordant cases were re-reviewed by a radiologist with expertise in prostate mpMRI to determine reason for discordance.
Results
A total of 521 lesions were identified on mpMRI. 121/521 (23.2%), 310/524 (59.5%), and 90/521 (17.3%) were PI-RADS 5, 4, and 3, respectively. PPV of PI-RADS 5, 4, and 3 for all PCa and csPCa was 0.80, 0.55, 0.24 and 0.63, 0.33, and 0.09, respectively. 45 cases of discordant biopsy results for PI-RADS 5 lesions were found with 27 deemed “true” discordances or “unresolved” discordances where imaging re-review confirmed PI-RADS appropriateness, while 18 were deemed “false” or resolved discordances due to downgrading of PI-RADS scores based on imaging re-review. Adjusting for resolved discordances on re-review, the PPV of PI-RADS 5 lesions for csPCa was deemed to be 0.74 and upon adjusting for presence of csPCa found in cases of unresolved discordance, PPV rose to 0.83 for PI-RADS 5 lesions.
Conclusion
Although PIRADS 5 lesions are considered high risk for csPCa, the PPV is not 100% and a diagnostic dilemma occurs when targeted biopsy returns discordant. While PI-RADS score is downgraded in some cases upon imaging re-review, a number of “false” or “unresolved” discordances were identified in which MRI re-review confirmed initial PI-RADS score and subsequent pathology confirmed presence of csPCa in these lesions.
Clinical impact
We propose a structured approach to resolving discordant biopsy results using multi-disciplinary re-review of imaging and archived biopsy strikes as a quality improvement pathway. Further work is needed to determine the value of re-biopsy in cases of unresolved discordance and to develop robust QI systems for prostate MRI.
Graphical abstract






Similar content being viewed by others

References
Albertsen PC, Hanley JA, Fine J (2005) 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 293 (17):2095-2101. https://doi.org/10.1001/jama.293.17.2095
Bill-Axelson A, Holmberg L, Garmo H, Taari K, Busch C, Nordling S, Haggman M, Andersson SO, Andren O, Steineck G, Adami HO, Johansson JE (2018) Radical Prostatectomy or Watchful Waiting in Prostate Cancer - 29-Year Follow-up. N Engl J Med 379 (24):2319-2329. https://doi.org/10.1056/NEJMoa1807801
Pokorny MR, de Rooij M, Duncan E, Schroder FH, Parkinson R, Barentsz JO, Thompson LC (2014) Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol 66 (1):22-29. https://doi.org/10.1016/j.eururo.2014.03.002
Lecornet E, Ahmed HU, Hu Y, Moore CM, Nevoux P, Barratt D, Hawkes D, Villers A, Emberton M (2012) The accuracy of different biopsy strategies for the detection of clinically important prostate cancer: a computer simulation. J Urol 188 (3):974-980. https://doi.org/10.1016/j.juro.2012.04.104
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM, Merino MJ, Simon RM, Choyke PL, Wood BJ, Pinto PA (2015) Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 313 (4):390-397. https://doi.org/10.1001/jama.2014.17942
Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, Bloom J, Gurram S, Siddiqui M, Pinsky P, Parnes H, Linehan WM, Merino M, Choyke PL, Shih JH, Turkbey B, Wood BJ, Pinto PA (2020) MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med 382 (10):917-928. https://doi.org/10.1056/NEJMoa1910038
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, Kirkham AP, Oldroyd R, Parker C, Emberton M, group Ps (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389 (10071):815-822. https://doi.org/10.1016/S0140-6736(16)32401-1
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Futterer JJ, European Society of Urogenital R (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22 (4):746-757. https://doi.org/10.1007/s00330-011-2377-y
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, Margolis D, Schnall MD, Shtern F, Tempany CM, Thoeny HC, Verma S (2016) PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 69 (1):16-40. https://doi.org/10.1016/j.eururo.2015.08.052
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, Tempany CM, Choyke PL, Cornud F, Margolis DJ, Thoeny HC, Verma S, Barentsz J, Weinreb JC (2019) Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol 76 (3):340-351. https://doi.org/10.1016/j.eururo.2019.02.033
Barkovich EJ, Shankar PR, Westphalen AC (2019) A Systematic Review of the Existing Prostate Imaging Reporting and Data System Version 2 (PI-RADSv2) Literature and Subset Meta-Analysis of PI-RADSv2 Categories Stratified by Gleason Scores. AJR Am J Roentgenol 212 (4):847-854. https://doi.org/10.2214/AJR.18.20571
Westphalen AC, McCulloch CE, Anaokar JM, Arora S, Barashi NS, Barentsz JO, Bathala TK, Bittencourt LK, Booker MT, Braxton VG, Carroll PR, Casalino DD, Chang SD, Coakley FV, Dhatt R, Eberhardt SC, Foster BR, Froemming AT, Futterer JJ, Ganeshan DM, Gertner MR, Mankowski Gettle L, Ghai S, Gupta RT, Hahn ME, Houshyar R, Kim C, Kim CK, Lall C, Margolis DJA, McRae SE, Oto A, Parsons RB, Patel NU, Pinto PA, Polascik TJ, Spilseth B, Starcevich JB, Tammisetti VS, Taneja SS, Turkbey B, Verma S, Ward JF, Warlick CA, Weinberger AR, Yu J, Zagoria RJ, Rosenkrantz AB (2020) Variability of the Positive Predictive Value of PI-RADS for Prostate MRI across 26 Centers: Experience of the Society of Abdominal Radiology Prostate Cancer Disease-focused Panel. Radiology 296 (1):76-84. https://doi.org/10.1148/radiol.2020190646
Meng X, Chao B, Chen F, Huang R, Taneja SS, Deng FM (2021) Followup of Men with PI-RADS 4 or 5 Abnormality on Prostate Magnetic Resonance Imaging and Nonmalignant Pathological Findings on Initial Targeted Prostate Biopsy. J Urol 205 (3):748-754. https://doi.org/10.1097/JU.0000000000001424
Rosenkrantz AB, Taneja SS (2014) Radiologist, be aware: ten pitfalls that confound the interpretation of multiparametric prostate MRI. AJR Am J Roentgenol 202 (1):109-120. https://doi.org/10.2214/AJR.13.10699
Li JL, Phillips D, Towfighi S, Wong A, Harris A, Black PC, Chang SD (2021) Second-opinion reads in prostate MRI: added value of subspecialty interpretation and review at multidisciplinary rounds. Abdom Radiol (NY). https://doi.org/10.1007/s00261-021-03377-1
Tay KJ, Gupta RT, Rastinehad AR, Tsivian E, Freedland SJ, Moul JW, Polascik TJ (2016) Navigating MRI-TRUS fusion biopsy: optimizing the process and avoiding technical pitfalls. Expert Rev Anticancer Ther 16 (3):303-311. https://doi.org/10.1586/14737140.2016.1131155
Tay KJ, Gupta RT, Brown AF, Silverman RK, Polascik TJ (2016) Defining the Incremental Utility of Prostate Multiparametric Magnetic Resonance Imaging at Standard and Specialized Read in Predicting Extracapsular Extension of Prostate Cancer. Eur Urol 70 (2):211-213. https://doi.org/10.1016/j.eururo.2015.10.041
Meng X, Rosenkrantz AB, Huang R, Deng FM, Wysock JS, Bjurlin MA, Huang WC, Lepor H, Taneja SS (2018) The Institutional Learning Curve of Magnetic Resonance Imaging-Ultrasound Fusion Targeted Prostate Biopsy: Temporal Improvements in Cancer Detection in 4 Years. J Urol 200 (5):1022-1029. https://doi.org/10.1016/j.juro.2018.06.012
Barrett T, Ghafoor S, Gupta RT, Kim CK, Muglia VF, Macura KJ, Purysko AS (2022) Prostate MRI Qualification: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.22.27615
de Rooij M, Israel B, Tummers M, Ahmed HU, Barrett T, Giganti F, Hamm B, Logager V, Padhani A, Panebianco V, Puech P, Richenberg J, Rouviere O, Salomon G, Schoots I, Veltman J, Villeirs G, Walz J, Barentsz JO (2020) ESUR/ESUI consensus statements on multi-parametric MRI for the detection of clinically significant prostate cancer: quality requirements for image acquisition, interpretation and radiologists' training. Eur Radiol 30 (10):5404-5416. https://doi.org/10.1007/s00330-020-06929-z
Barrett T, Padhani AR, Patel A, Ahmed HU, Allen C, Bardgett H, Belfield J, Brizmohun Appayya M, Harding T, Hoch OS, Keanie JY, Liyanage SH, Papoutsaki MV, Punwani S, Robinson MJC, Rajesh A, Stafurth JN, van der Meulen J, Richenberg J (2021) Certification in reporting multiparametric magnetic resonance imaging of the prostate: recommendations of a UK consensus meeting. BJU Int 127 (3):304-306. https://doi.org/10.1111/bju.15285
Brizmohun Appayya M, Adshead J, Ahmed HU, Allen C, Bainbridge A, Barrett T, Giganti F, Graham J, Haslam P, Johnston EW, Kastner C, Kirkham APS, Lipton A, McNeill A, Moniz L, Moore CM, Nabi G, Padhani AR, Parker C, Patel A, Pursey J, Richenberg J, Staffurth J, van der Meulen J, Walls D, Punwani S (2018) National implementation of multi-parametric magnetic resonance imaging for prostate cancer detection - recommendations from a UK consensus meeting. BJU Int 122 (1):13-25. https://doi.org/10.1111/bju.14361
Funding
This project was performed at the Departments of Radiology, Pathology and Surgery at Duke University Medical Center and with support of the Duke Cancer Institute Center for Prostate and Urologic Cancers. There is no external or internal funding for this project. This manuscript is not under consideration elsewhere.
Author information
Authors and Affiliations
Contributions
Specific ICMJE contributions by author for requirements as above (in addition to final approval of version to be published which was done by all authors): RA, M.D. – data acquisition, analysis, and interpretation of data, manuscript preparation. SS, B.S. – data acquisition, analysis, and interpretation of data, manuscript preparation. SK, M.D. – data acquisition, analysis, and interpretation of data, manuscript preparation. MK, B.S. – interpretation of data, manuscript preparation. ZDM, B.S., M.S. – data acquisition, analysis, and interpretation of data, manuscript preparation. W-CF, M.D.. – data acquisition, manuscript preparation, histopathologic image acquisition. JH, M.D., Ph.D.. – -data acquisition, manuscript preparation, histopathologic image acquisition. TJP, M.D., FACS. – data acquisition, analysis, and interpretation of data, manuscript preparation. RTG, M.D.. – data acquisition, analysis, and interpretation of data, manuscript preparation, guarantor of manuscript and corresponding author.
Corresponding author
Ethics declarations
Conflict of interest
Rajan T. Gupta, MD – Consultant, Invivo Corp. No other financial disclosures for all other authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arcot, R., Sekar, S., Kotamarti, S. et al. Structured approach to resolving discordance between PI-RADS v2.1 score and targeted prostate biopsy results: an opportunity for quality improvement. Abdom Radiol 47, 2917–2927 (2022). https://doi.org/10.1007/s00261-022-03562-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03562-w