Abstract
Background
Gastrointestinal complications of coronavirus disease-2019 (COVID-19) include abnormal liver function and acalculous cholecystitis. Cholecystostomy performed during the COVID-19 pandemic reflected a shift toward non-surgical treatment of cholecystitis and increased number of critically ill patients suffering from acalculous cholecystitis.
Purpose
(1) To determine demographic, clinical, laboratory, and ultrasound features associated with cholecystostomy placement during hospitalization for COVID-19. (2) To develop multivariable logistic regression modeling for likelihood of biliary intervention.
Methods
This retrospective review received institutional review board approval. Informed consent was waived. Between March 2020 and June 2020, patients with confirmed SARS-CoV2 infection admitted to New York-Presbyterian Hospital (NYP)/Weill Cornell Medical Center, NYP/Lower Manhattan Hospital, and NYP/Queens were evaluated for inclusion in this study. Inclusion criteria were (1) patient age ≥ 18, (2) confirmed COVID-19 infection by polymerase chain reaction testing of a nasopharyngeal swab, and (3) abdominal ultrasound performed during hospitalization. Exclusion criteria were (1) history of cholecystectomy and (2) biliary intervention performed prior to abdominal ultrasound. Patients were stratified into two groups based on whether they received cholecystostomy during hospitalization. Differences in demographics, medical history, clinical status, medications, laboratory values, and ultrasound findings between the two groups were evaluated using Chi-square test or Fisher’s exact test for categorical variables and t test or Wilcoxon-rank sum test for continuous variables. Multivariable logistic regression was used to model likelihood of biliary intervention.
Results
Nine patients underwent cholecystostomy placement and formed the “Intervention Group.” 203 patients formed the “No Intervention Group.” Liver size and diuretics use during hospitalization were the only variables which were significantly different between the two groups, with p-values of 0.02 and 0.046, respectively. After controlling for diuretics use, the odds of receiving cholecystostomy increased by 30% with every centimeter increase in liver size (p = 0.03). ICU admission approached significance (p = 0.16), as did mechanical ventilation (p = 0.09), septic shock (p = 0.08), serum alkaline phosphatase level (p = 0.16), and portal vein patency (0.14).
Conclusion
Patients requiring biliary intervention during hospital admission for COVID-19 were likely to harbor liver injury in the form of liver enlargement and require diuretics use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
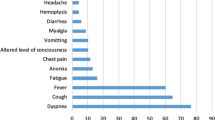
Coronavirus disease-2019 (COVID-19), a pandemic caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), has resulted in more than 289 million cases and over 5.4 million deaths worldwide as of January 2022 [1]. Although mortality and morbidity related to COVID-19 are mostly attributed to lung involvement, many patients suffer hepatobiliary injury as well. Abnormal liver function tests are frequently seen in patients hospitalized for COVID-19 and represent the most common gastrointestinal complication in COVID-19 patients, followed by acute acalculous cholecystitis [2,3,4,5,6]. In a cohort of critically ill, COVID-19 patients, transaminitis, and acalculous cholecystitis accounted for 67% and 4% of gastrointestinal complications, respectively [3].
During the first surge of the COVID-19 pandemic in early 2020, effective patient and resource management ensuring the safety of both the patients and healthcare providers represented an unprecedented challenge. Percutaneous cholecystostomy tube placement, traditionally performed for treatment of acalculous cholecystitis or as a bridge to laparoscopic cholecystectomy in patients who are poor surgical candidates, had a relative increase in volume [7,8,9,10]. This trend likely reflected both a shift toward non-surgical treatment of acute cholecystitis during the pandemic, as well as an increase in the number of critically ill patients on mechanical ventilation, which itself is a risk factor for acalculous cholecystitis due to biliary stasis and gallbladder ischemia [11]. Other predisposing factors for acalculous cholecystitis include hyperalimentation and prolonged fasting, which are also commonly seen in critically ill patients [11].
The purpose of this study is to determine demographic, clinical, laboratory, and ultrasound features associated with cholecystostomy tube placement during hospital admission for COVID-19 and to develop a multivariable logistic regression model to evaluate the likelihood of biliary intervention. A secondary purpose of this study is to evaluate the efficacy of abdominal ultrasound in triaging patients for biliary interventions and to determine which ultrasound findings are not useful, which could potentially abbreviate the conventional abdominal ultrasound protocol, thereby reducing technologist/patient contact time during a period of active COVID-19 infection.
Methods
Patient population
This retrospective review of existing clinical data received institutional review board approval at Weill Cornell Medical Center. Informed consent was waived. Between March 3, 2020 and June 5, 2020, all patients with confirmed SARS-CoV2 infection who were admitted to New York-Presbyterian Hospital (NYP)/Weill Cornell Medical Center, NYP/Lower Manhattan Hospital, and NYP/Queens were identified in the clinical database and evaluated for inclusion in this study. Patients were included in the study based on the following inclusion criteria: (1) patient age ≥ 18, (2) confirmed COVID-19 infection by positive results on polymerase chain reaction testing of a nasopharyngeal swab, and (3) abdominal ultrasound performed during hospital admission based on clinical signs/symptoms and laboratory values as determined by the clinical team. Exclusion criteria were (1) history of cholecystectomy and (2) biliary intervention performed prior to abdominal ultrasound.
Patient demographics (age and sex), medical history, medications, laboratory values, and abdominal ultrasound findings were extracted from the electronic medical database using the NYP COVID-19 research data repository. Clinical status including vital signs, ventilation status, co-infection, and hospital acquired complications was recorded. Hospital acquired complications encompassed conditions, such as acute renal injury, septic shock, ventilator-associated pneumonia, and myocardial infarction which developed during the course of hospitalization. Laboratory results, including serum total and direct bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase, albumin, prothrombin time (PT), partial thromboplastin time (PTT), white blood cell count (WBC), and neutrophil counts, were obtained within 24 h of the abdominal ultrasound exam.
Electronic medical database was used to identify patients who had interventional radiology (IR)-guided cholecystostomy tube placement following abdominal ultrasound during hospital admission. The date of intervention, appearance of the bile aspirate, improvement in leukocytosis, and liver function tests were recorded. Lastly, patients who died during hospital admission for COVID-19 were noted.
Abdominal ultrasound
Abdominal ultrasound was performed using General Electric Medical Systems Logic S8 Portable machines or GE E10, using a 3–4-MHz curved transducer. Registered diagnostic medical sonographers (RDMS) with one to 13 years of experience acquired images portably at the bedside or in the ultrasound suite as per American Institute of Ultrasound in Medicine (AIUM) guidelines. Images were interpreted by 20 board-certified radiologists with 2–24 years of experience. The scanning protocol included transverse and longitudinal gray-scale images of the right and left lobes of the liver and gallbladder, color Doppler images of the hepatic and portal veins, and gray-scale image of the common bile duct. A 9-MHz linear transducer was used to evaluate liver surface contour.
Ultrasound features extracted from the reports included liver size (longitudinal dimension), smooth versus non-smooth liver surface contour, normal versus increased liver echogenicity, portal and hepatic vein patency, gallbladder wall thickness, presence of pericholecystic fluid, sonographic Murphy’s sign, biliary ductal dilatation, common hepatic duct diameter, and presence of ascites.
Statistical analysis
Patients were stratified into two groups based on whether they received biliary intervention during hospital admission. As initial analysis, differences in demographics, medical history, clinical status, medications, laboratory values, and ultrasound findings between the two groups were evaluated using Chi-square test or Fisher’s exact test (as appropriate) for categorical variables and using t test or Wilcoxon-rank sum test (as appropriate) for continuous variables. Continuous variables were reported as median and interquartile range (IQR). Categorical variables were reported with frequency and percent. Subsequently, multivariable logistic regression was used to model likelihood of biliary intervention where biliary intervention was the binary outcome variable, and variables found to be significant in the initial analysis were used as independent variables. Collinearity of the significant variables was assessed by calculating variance inflation factors. The numerical value for VIF indicates what percentage the variance (i.e., the standard error squared) is inflated for each coefficient. 1 = not correlated. Between 1 and 5 = moderately correlated. Greater than 5 = highly correlated.
Results
Between March 3, 2020 and June 5, 2020, 254 admitted patients with confirmed SARS-CoV2 infection underwent abdominal ultrasound. Thirty-seven patients with history of cholecystectomy and one patient who underwent ERCP with stone extraction during hospital admission were excluded from the study. Two patients were excluded as their ultrasounds were performed after biliary intervention. Two pediatric patients under the age of 18 were also excluded. In total, 212 COVID-19 patients were included in the study, consisting of 148 men and 64 women, with median age of 70.5 years (range 22–101 years).
Nine patients received biliary intervention, specifically IR-guided percutaneous cholecystostomy placement during hospital admission, forming the “Intervention Group” (Figs. 1, 2). On average, patients underwent cholecystostomy placement 2.8 days after completion of abdominal ultrasound (range 0–9 days). Five patients were noted to have dark turbid bile aspirate during cholecystostomy. Bile aspirate was not characterized in the remaining 4 patients. Improvement in leukocytosis and liver function tests was observed in 5 patients (56%) and 6 patients (67%), respectively.
49-year-old woman with COVID-19 infection and transaminitis. a Abdominal ultrasound demonstrated sludge within a distended gallbladder (star) with gallbladder wall thickening measuring 5 mm (black arrow), concerning for acalculous cholecystitis. b Percutaneous cholecystostomy was performed via transhepatic approach (white arrow). After prolonged hospital course lasting three months, the patient was discharged in stable condition
60-year-old woman with COVID-19 infection, elevated white blood cell count and abdominal pain. a Abdominal ultrasound demonstrated findings of acalculous cholecystitis, including sludge within a distended gallbladder (star), gallbladder wall thickening and pericholecystic fluid (white arrow). b Patient underwent percutaneous cholecystostomy (black arrow) and was eventually discharged home following a two-month stay in the intensive care unit
The remaining 203 patients who did not undergo biliary intervention formed the “No Intervention Group.” Of all the demographic, clinical, laboratory and ultrasound variables, liver size, and diuretics use during hospitalization were the only variables which were significantly different between the two groups, with p-values of 0.02 and 0.046, respectively (Tables 1, 2, 3). Patients in the “Intervention Group” demonstrated greater liver size on abdominal ultrasound with median size of 19.3 cm (IQR 16.8–21.9 cm) versus “No Intervention Group” with median liver size of 16.4 cm (IQR 15.3–18.2 cm). A greater percentage of patients requiring biliary intervention were on diuretics (n = 8, 89%), compared with the “No Intervention Group” (n = 110, 54%).
ICU admission approached significance (p = 0.16), as did mechanical ventilation (p = 0.09), septic shock (p = 0.08), serum alkaline phosphatase level (p = 0.16), and portal vein patency (p = 0.14) between the two groups (Tables 2, 3). Specifically, a greater percentage of patients in the “Intervention Group” required ICU admission (n = 8, 89% versus n = 124, 61% in “No Intervention Group”), mechanical ventilation (n = 8, 89% versus n = 121, 60% in “No Intervention Group”), and had documented septic shock (n = 4, 44% versus n = 39, 19% in “No Intervention Group”). Elevations in alkaline phosphatase were higher in patients requiring biliary intervention (median 253 units/L, IQR 123–301 units/L), compared with those who did not require biliary intervention (median 150 units/L, IQR 83–260 units/L). More patients in the “No Intervention Group” demonstrated portal vein patency (n = 189, 93%), compared with the “Intervention Group” (n = 7, 78%).
There was no significant difference between the two groups in terms of patient demographics, past medical history, vital signs, or any of the remaining clinical, medication, laboratory, or imaging variables (Tables 1, 2, 3). A higher death rate was observed in the “Intervention Group” (n = 5, 56%), compared with the “No Intervention Group” (n = 48, 24%, p = 0.04).
Multivariable logistic regression was performed using the two significant co-variates, namely liver size and diuretics use during hospitalization. After controlling for the use of diuretics, the odds of receiving biliary intervention increased by 30% with every centimeter increase in liver size (odds ratio 1.3; 95% confidence interval 1.07–1.69; p = 0.03). Variance inflation factors (VIF) were calculated to assess the collinearity of these significant variables. VIF of liver size and diuretics use were close to 1 (1.001552 and 1.060848, respectively), supporting the fact that variables in the multivariable logistic regression were not co-linear.
Discussion
Cholecystitis is an important complication in hospitalized COVID-19 patients and, in this series, required cholecystostomy in 9 of 212 patients (4%) undergoing abdominal ultrasound. Liver size was significantly different between patients who ultimately underwent biliary intervention and those who did not, suggesting that those who required cholecystostomy had severe liver injury enlarging the liver [12]. After controlling for diuretics use (a significant covariate), we found that with every centimeter increase in liver size, the odds of receiving biliary intervention increased significantly by 30%.
A number of mechanisms have been postulated for COVID-19-related hepatobiliary disease, including direct viral infection via ACE2 receptors on hepatocytes and cholangiocytes, immune mediated as a result of systematic inflammatory response syndrome (SIRS), hypoxemia, vasculitis, and drug-induced injury [5, 13]. On histology, COVID-related liver injury is characterized by hepatic steatosis, microthromboses in the portal vein branches and sinusoids, portal inflammation, and fibrosis [14]. Studies have reported a biphasic pattern in COVID-19 patients with an initial rise in AST and ALT, followed by elevations in alkaline phosphatase and bilirubin, which may reflect bile duct injury later in the stage of the infection [15, 16]. We observed similar elevations in AST, ALT, alkaline phosphatase, total and direct bilirubin in our COVID-19 patient population, as well as prolonged prothrombin time and decreased albumin levels, reflecting decreased synthetic function of the liver. Of the laboratory variables, only differences in serum alkaline phosphatase elevations approached significance between the “Intervention Group” and “No Intervention Group” (p = 0.16), suggesting that patients requiring biliary intervention tended to exhibit a cholestatic pattern of hepatobiliary injury.
We also observed that patients who underwent cholecystostomy tube placement were generally more ill, given a higher percentage of patients in the “Intervention Group” requiring ICU admission and mechanical ventilation and with documented septic shock, likely contributing to the higher death rate observed in this group. Acalculous cholecystitis is a known complication of critical illness and mechanical ventilation with high mortality rate of 30–50% [11, 17, 18]. Prompt diagnosis and treatment consisting of cholecystostomy drainage are necessary to prevent gallbladder gangrene, perforation, and sepsis [11, 17]. Ultrasound remains the diagnostic test of choice in patients suspected of having cholecystitis given its high sensitivity, specificity, portability, lack of ionizing radiation, and low cost [18]. Imaging findings of acalculous cholecystitis include gallbladder distention, wall thickening, pericholecystic fluid, and sonographic Murphy’s sign [18].
Unexpectedly, none of the ultrasound features other than increased liver size was associated with likelihood of subsequent biliary intervention. Interestingly, a slightly lower percentage of patients who required cholecystostomy had documented portal vein patency. Our findings suggest that although acalculous cholecystitis is traditionally diagnosed with abdominal ultrasound, none of the gallbladder-specific ultrasound features were critical in triaging patients for biliary intervention; a patient’s clinical status, such as critical illness, septic shock, diuretics use, and alkaline phosphatase elevations, may have a greater role in this context. These results are important in both resource and patient management for future COVID surges. Patients with a high pre-test probability of undergoing biliary intervention based on clinical factors may benefit from expedited pre-procedure preparation without delay from an abdominal ultrasound. Also, while abbreviating standard ultrasound protocol during a pandemic would decrease patient contact time and risk of infection to the ultrasound staff, liver size measurement remains crucial in patients suspected of acalculous cholecystitis and should be performed regardless of the sonographic appearance of the gallbladder.
During the study period, operative interventions and aerosolizing procedures were severely limited due to the COVID-19 response, with many operating rooms being closed or converted to ICUs. Although operative access was severely limited during the early phase of the COVID-19 pandemic, we believe that our results can be referenced during subsequent waves of the COVID-19 pandemic when surgeries are not limited for the following reasons. First, majority of patients who underwent cholecystostomy tube placement in this study were ICU patients on mechanical ventilation (n = 8, 89%), who were likely non-surgical candidates and would require temporizing treatment such as cholecystostomy rather than cholecystectomy. Secondly, although disease-modifying treatments were not readily available during the first wave of COVID-19 as they are today, vaccination was also not available during the first wave. Similarly, majority of hospitalized and critically ill COVID-19 patients today are also unvaccinated. Therefore, to a certain degree, our data can be generalized to the present time.
The early COVID-19 response with limited operative access may explain the seemingly longer wait times between abdominal ultrasound and biliary intervention in our series, i.e., average of 2.8 days for cholecystostomy. It is possible that ultrasound findings might have evolved during the time between the abdominal ultrasound and biliary intervention; however, given the retrospective nature of the study, we could not require a specific time interval between the two procedures. Other limitations of this study include selection bias as we included only COVID-positive patients imaged with abdominal ultrasound, which was performed based on clinical or laboratory concerns during hospital admission. Absolute longitudinal liver size was used in the analysis without correcting for patient’s sex or body habitus, which could be confounding, although longitudinal dimension of the liver was consistently measured in a standard fashion for all patients in both groups in this study. Our overall sample size was small, as was the number of patients requiring biliary intervention. The unbalanced number of subjects in the intervention group reduced the statistical power of the study. Future studies with a larger sample size might identify additional predictive factors and could be used to create more sophisticated multivariable modeling for prediction of biliary intervention.
In conclusion, patients who required biliary intervention during hospital admission for COVID-19 infection were likely to harbor liver injury in the form of liver enlargement and require diuretics use. Aside from liver size measurement, abdominal ultrasound may not be an effective tool in triaging patients for biliary intervention.
References
COVID-19 Map - Johns Hopkins Coronavirus Resource Center. 2021 [Available from: https://coronavirus.jhu.edu/map.html
Bertolini A, van de Peppel IP, Bodewes F, Moshage H, Fantin A, Farinati F, et al. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72(5):1864-72.
Kaafarani HMA, El Moheb M, Hwabejire JO, Naar L, Christensen MA, Breen K, et al. Gastrointestinal Complications in Critically Ill Patients With COVID-19. Ann Surg. 2020;272(2):e61-e2.
Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52(4):584-99.
Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41(1):20-32.
Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, et al. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol. 2021;21.
Barabino M, Piccolo G, Trizzino A, Fedele V, Ferrari C, Nicastro V, et al. COVID-19 outbreak and acute cholecystitis in a Hub Hospital in Milan: wider indications for percutaneous cholecystostomy. BMC Surg. 2021;21(1):180.
Mori Y, Itoi T, Baron TH, Takada T, Strasberg SM, Pitt HA, et al. Tokyo Guidelines 2018: management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):87-95.
Patel M, Miedema BW, James MA, Marshall JB. Percutaneous cholecystostomy is an effective treatment for high-risk patients with acute cholecystitis. Am Surgeon. 2000;66(1):33-7.
Snyder A, Salamone S, Reid NJ, Yeung T, Di Capua J, Som A, et al. Retrospective evaluation of image-guided cholecystostomy tube utilization and outcomes during the first wave of the COVID-19 pandemic. American Journal of Interventional Radiology. 2021;5(13).
Barie PS, Eachempati SR. Acute acalculous cholecystitis. Gastroenterol Clin North Am. 2010;39(2):343–57, x.
Lala V, Goyal A, Bansal P, Minter DA. Liver Function Tests. StatPearls. Treasure Island (FL)2021.
Zhao B, Ni C, Gao R, Wang YY, Yang L, Wei JS, et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771-5.
Marjot T, Webb GJ, Barritt ASt, Moon AM, Stamataki Z, Wong VW, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18(5):348–64.
Bernal-Monterde V, Casas-Deza D, Letona-Gimenez L, de la Llama-Celis N, Calmarza P, Sierra-Gabarda O, et al. SARS-CoV-2 Infection Induces a Dual Response in Liver Function Tests: Association with Mortality during Hospitalization. Biomedicines. 2020;8(9).
Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, et al. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116(5):1077-82.
Balmadrid B. Recent advances in management of acalculous cholecystitis. F1000Res. 2018;7.
Chawla A, Bosco JI, Lim TC, Srinivasan S, Teh HS, Shenoy JN. Imaging of acute cholecystitis and cholecystitis-associated complications in the emergency setting. Singapore Med J. 2015;56(8):438–43; quiz 44.
Acknowledgements
This study received support from New York-Presbyterian Hospital (NYPH) and Weill Cornell Medical College (WCMC), including the Clinical and Translational Science Center (CTSC) (UL1 TR000457) and Joint Clinical Trials Office (JCTO).
Funding
This study received support from New York-Presbyterian Hospital (NYPH) and Weill Cornell Medical College (WCMC), including the Clinical and Translational Science Center (CTSC) (UL1 TR000457) and Joint Clinical Trials Office (JCTO). No relevant disclosures from the corresponding author and co-authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, C., Riyahi, S., Prince, M. et al. Predictors of biliary intervention in patients hospitalized for COVID-19. Abdom Radiol 47, 1891–1898 (2022). https://doi.org/10.1007/s00261-022-03461-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03461-0