Abstract
Purpose
To explore the value of various diffusion parameters obtained from monoexponential, biexponential, and stretched exponential in assessing liver fibrosis in chronic hepatitis B (CHB).
Methods
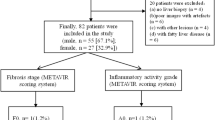
DWI and intravoxel incoherent motion (IVIM) MRI were performed prospectively on liver for 146 patients with CHB and 21 healthy volunteers. ADC values were obtained from monoexponential model imaging. Diffusion coefficient (D), pseudodiffusion coefficient (D*), and perfusion fraction (f) obtained by biexponential model imaging, and stretched exponential model to obtain diffusion distribution coefficient (DDC) and diffusion heterogeneity index (α). Blood draw were performed on patients to obtain AST, ALT, and PLT, and then APRI and FIB-4 index were determined based on the serological diagnostic models. The fibrosis stage was staged (S0–S4) according to the pathology of liver puncture. Independent sample t test was used to compare the parameter values between liver fibrosis group and control group. One-way ANOVA was used to compare the parameters of different liver fibrosis grades. Bonferroni test was used for correcting multiple comparisons. Spearman correlation was used to analyze the correlation between each parameter and liver fibrosis grades. ROC was used to predict the diagnostic power of each parameter for liver fibrosis stages ≥ S2 and ≥ S3.
Results
ADC, D, D*, f, and DDC values were significantly different between normal control group and hepatic fibrosis group (P < 0.05). There were significant differences in ADC, D*, f, and DDC value among liver fibrosis groups (P < 0.05). D* and DDC values were moderately negatively correlated with the grades of liver fibrosis (r = − 0.483, P < 0.001; r = − 0.622, P < 0.001). ADC and f values were slightly negatively correlated with the grades of liver fibrosis (r = − 0.295, P < 0.001; r = − 0.312, P < 0.001). DDC values have the highest diagnostic efficiency in liver fibrosis stages ≥ S2 and ≥ S3. The areas under ROC curve (AUC) were 0.813 and 0.832 for ≥ S2 and ≥ S3, respectively, the sensitivity is 83.72% and 73.53%, and the specificity of 83.33% and 66.04%, which were better than APRI and FIB-4.
Conclusion
D* obtained from biexponential and DDC obtained from stretched exponential DWI have better value in evaluating the degree of liver fibrosis in CHB.





Similar content being viewed by others
References
Liaw YF, Kao JH, Piratvisuth T, et al. Erratum to: Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update[J]. Hepatol Int, 2012, 6(4): 809-810. https://doi.org/10.1007/s12072-012-9386-z
Chow AM, Gao DS, Fan SJ, et al. Liver fibrosis: an intravoxel incoherent motion (IVIM) study[J]. Magn Reson Imaging, 2012, 36(1): 159-167.PMID: 22334528.
Shi Y, Guo QY, Fu XX, et al. Standardized apparent diffusion coefficient of 3.0T MR in evaluating the degree of liver fibrosis [J]. Chin J Radiol, 2012,46: 322–326.
Cece H, Ercan A, Yιldιz S, et al. The use of DWI to assess spleen and liver quantitative ADC changes in the detection of liver fibrosis stages in chronic viral hepatitis[J]. Eur J Radiol, 2013, 82(8): e307-e312.
Zou LQ, Pan J, Cheng XY, et al. Value of MR susceptibility-weighted imaging in the diagnosis of hepatic fibrosis grading in rabbits [J].Chin J Radiol, 2015,49 (8): 615–618.
Fan GH, Gong JP, Shen JK, et al. Value of MR diffusion-weighted imaging in diagnosis of liver fibrosis in rats [J]. Chin J Radiol, 2013,47 (2): 172-177.
Koh DM, Collins DJ, Orton MR, et al. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges[J]. AJR, 2011,196(6):1351-1361.
Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003. 226(1): 71-8
Chinese Society of Hepatology, Chinese Society of Infectious Diseases, Chinese Medical Association. Guideline for prevention and treatment of chronic hepatitis B [J]. Chinese Journal of Hepatology: Electronic Edition, 2015,7 (3): 1–18.
Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV confection[J]. Hepatology, 2006,43:1317-1325.
Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C[J]. Hepatology, 2003,38:518-526.
Kumar M, Sarin SK. Is cirrhosis of the liver reversible[J] Indian J Pediatr,2007,74(4): 393–399.
Luciani A, Vignaud A, Cavet M, Nhieu JT, Mallat A, Ruel L, et al. Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study[J]. Radiology. 2009,249(3):891-9.
Huseyin O, Firat K, Ozan K, et al. Diffusion Weighted MRI for Hepatic Fibrosis: impact of b-Value. Iran J Radiol, 2014, 11(1): e3555.
Klauss M, Mayer P, Maier-Hein K, etal. IVIM-diffusion-MRI for the differentiation of solid begin and malign hypervascular liver lesions-evaluation with two different MR scanner[J]. EurJ Radiol,2016,85(7):1289-1294.
Tokgoz O, Unal I, Turgut GG, et al. The value of liver and spleen ADC measurements in the diagnosis and follow up of hepatic fibrosis in chronic liver disease. Acta clinica Belgica, 2014, 69(6): 426-432.
Amona L, Wurnig MC, Luechinger R, et al. Whole-body intravoxel incoherent motion imaging [J]. Eur Radiol,2015,25(7):2049-2058.
Lazar M, Jensen JH, Xuan L, Helpern JA. Estimation of the orientation distribution function from diffusional kurtosis imaging. Magn Reson Med. 2008. 60(4): 774-81.
Cohen Y, Assaf Y. High b-value q-space analyzed diffusion-weighted MRS and MRI in neuronal tissues - a technical review. NMR Biomed. 2002. 15(7-8): 516-542.
Anderson SW, Barry B, Soto J, Ozonoff A, O'Brien M, Jara H. Characterizing non-gaussian, high b-value diffusion in liver fibrosis: Stretched exponential and diffusional. 39(4): 827–34.
Xie Lf, Liang Ch. Research progress of intratosomal incoherent motor imaging in liver [J]. Chinese journal of radiology. 2014,48 (1): 77-9.
Le Bihan D, Turner R, Macfall JR, et al. Effects of intravoxel incoherent motions (IVIM) in steady-state free precession (SSFP) imaging: application to molecular diffusion imaging[J]. Magn Reson Med, 1989, 10(3): 324-337.PMID: 2733589.
Zeng Z, Lu BP, Huang H. The preliminary study on the diagnosis of liver fibrosis with intra-voxel incoherent motion imaging [J]. Radiology Practice. 2015 (7): 775-8.
Walter SS, Liu W, Stemmer A, et al. Combination of integrated dynamic shimming and readout-segmented echo planar imaging for diffusion weighted MRI of the head and neck region at 3 Tesla. Magn Reson Imaging, 2017, 42: 32-36.
Li C, Zhu SC, Zhang DX, et al. Value of intra-voxel incoherent motion diffusion-weighted MR imaging in evaluating the degree of liver fibrosis in patients with chronic hepatitis B. Magnetic Resonance Imaging, 2017,8 (9): 662-667.
Andreou A, Koh DM, Collins DJ, et al. Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases[J]. Eur Radial,2012,23:428-434
Sigmund EE, Vivier PH, Sui D, et al. Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges[J]. Radiology,2012,263.
Orton MR, Collins DJ, Koh DM, Leach MO. Improved intravoxel incoherent motion analysis of diffusion weighted imaging by data driven Bayesian modeling. Magn Reson Med. 2014;71(1):411-420.
Funding
Beijing Municipal Science & Technology Commission (Z181100001718006).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, H., Liu, Y., Lu, J. et al. Evaluating the clinical value of MRI multi-model diffusion-weighted imaging on liver fibrosis in chronic hepatitis B patients. Abdom Radiol 46, 1552–1561 (2021). https://doi.org/10.1007/s00261-020-02806-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02806-x