Abstract
Purpose
This paper discusses the optimization of pharmacokinetic modelling and alternate simplified quantification method for [18F]AlF-P16-093, a novel tracer for in vivo imaging of prostate cancer.
Methods
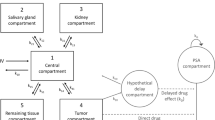
Dynamic PET/CT scans were conducted on eight primary prostate cancer patients, followed by a whole-body scan at 60 min post-injection. Time-activity curves (TACs) were obtained by drawing volumes of interest for primary prostatic and metastatic lesions. Optimal kinetic modelling involved evaluating three compartmental models (1T2K, 2T3K, and 2T4K) accounting for fractional blood volume (Vb). The simplified quantification method was then determined based on the correlation between the static uptake measure and total distribution volume (Vt) obtained from the optimal pharmacokinetic analysis.
Results
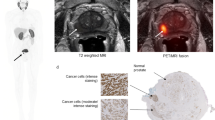
In total, 17 intraprostatic lesions, 10 lymph nodes, and 36 osseous metastases were evaluated. Visually, the contrast of the tumor increased and showed the steepest incline within the first few minutes, whereas background activity decreased over time. Full pharmacokinetic analysis revealed that a reversible two-compartmental (2T4K) model is the preferred kinetic model for the given tracer. The kinetic parameters K1, k3, Vb, and Vt were all significantly higher in lesions when compared with normal tissue (P < 0.01). Several simplified protocols were tested for approximating comprehensive dynamic quantification in tumors, with image-based SURmean (the ratio of tumor SUVmean to blood SUVmean) within the 28–34 min window found to be sufficient for approximating the total distribution Vt values (R2 = 0.949, P < 0.01). Both Vt and SURmean correlated significantly with the total serum prostate-specific antigen (tPSA) levels (P < 0.01).
Conclusions
This study introduced an optimized pharmacokinetic modelling approach and a simplified acquisition method for [18F]AlF-P16-093, a novel PSMA-targeted radioligand, highlighting the feasibility of utilizing one static PET imaging (between 30 and 60 min) for the diagnosis of prostate cancer. Note that the image-derived input function in this study may not reflect the true corrected plasma input function, therefore the interpretation of the associated kinetic parameter estimates should be done with caution.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, et al. Prostate cancer. Nat Rev Dis Prim. 2021;7:9. https://doi.org/10.1038/s41572-020-00243-0.
Zhang H, Koumna S, Pouliot F, Beauregard JM, Kolinsky M. PSMA Theranostics: current landscape and future outlook. Cancers. 2021;13. https://doi.org/10.3390/cancers13164023.
Carlucci G, Ippisch R, Slavik R, Mishoe A, Blecha J, Zhu S. (68)Ga-PSMA-11 NDA approval: a novel and successful academic partnership. J Nucl Med. 2021;62:149–55. https://doi.org/10.2967/jnumed.120.260455.
Panebianco V, Villeirs G, Weinreb JC, Turkbey BI, Margolis DJ, Richenberg J, et al. Prostate magnetic resonance imaging for local recurrence reporting (PI-RR): international consensus -based guidelines on multiparametric magnetic resonance imaging for prostate cancer recurrence after radiation therapy and radical prostatectomy. Eur Urol Oncol. 2021;4:868–76. https://doi.org/10.1016/j.euo.2021.01.003.
Tomasi G, Turkheimer F, Aboagye E. Importance of quantification for the analysis of PET data in oncology: review of current methods and trends for the future. Mol Imaging Biol. 2012;14:131–46. https://doi.org/10.1007/s11307-011-0514-2.
Dimitrakopoulou-Strauss A, Pan L, Sachpekidis C. Kinetic modeling and parametric imaging with dynamic PET for oncological applications: general considerations, current clinical applications, and future perspectives. Eur J Nucl Med Mol Imaging. 2021;48:21–39. https://doi.org/10.1007/s00259-020-04843-6.
Sachpekidis C, Eder M, Kopka K, Mier W, Hadaschik BA, Haberkorn U, et al. Ga-PSMA-11 dynamic PET/CT imaging in biochemical relapse of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:1288–99. https://doi.org/10.1007/s00259-015-3302-4.
Ringheim A, Campos Neto GC, Anazodo U, Cui L, da Cunha ML, Vitor T, et al. Kinetic modeling of (68)Ga-PSMA-11 and validation of simplified methods for quantification in primary prostate cancer patients. EJNMMI Res. 2020;10:12. https://doi.org/10.1186/s13550-020-0594-6.
Strauss DS, Sachpekidis C, Kopka K, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A. Pharmacokinetic studies of [(68) Ga]Ga-PSMA-11 in patients with biochemical recurrence of prostate cancer: detection, differences in temporal distribution and kinetic modelling by tissue type. Eur J Nucl Med Mol Imaging. 2021;48:4472–82. https://doi.org/10.1007/s00259-021-05420-1.
Sachpekidis C, Eder M, Kopka K, Mier W, Hadaschik BA, Haberkorn U, et al. (68)Ga-PSMA-11 dynamic PET/CT imaging in biochemical relapse of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:1288–99. https://doi.org/10.1007/s00259-015-3302-4.
Sachpekidis C, Afshar-Oromieh A, Kopka K, Strauss DS, Pan L, Haberkorn U, et al. (18)F-PSMA-1007 multiparametric, dynamic PET/CT in biochemical relapse and progression of prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:592–602. https://doi.org/10.1007/s00259-019-04569-0.
Malaspina S, Oikonen V, Kuisma A, Ettala O, Mattila K, Boström PJ, et al. Kinetic analysis and optimisation of (18)F-rhPSMA-7.3 PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:3723–31. https://doi.org/10.1007/s00259-021-05346-8.
Zha Z, Ploessl K, Choi SR, Wu Z, Zhu L, Kung HF. Synthesis and evaluation of a novel urea-based 68Ga-complex for imaging PSMA binding in tumor. Nucl Med Biol. 2018;59:36–47. https://doi.org/10.1016/j.nucmedbio.2017.12.007.
Lee H, Scheuermann JS, Young AJ, Doot RK, Daube-Witherspoon ME, Schubert EK, et al. Preliminary evaluation of 68Ga-P16-093, a PET radiotracer targeting prostate-specific membrane antigen in prostate cancer. Mol Imaging Biol. 2022;26:1–11. https://doi.org/10.1007/s11307-022-01720-6.
Wang G, Li L, Zang J, Hong H, Zhu L, Kung HF, et al. Head-to-head comparison of 68Ga-P16-093 and 68Ga-PSMA-617 PET/CT in patients with primary prostate cancer: a pilot study. Clin Nucl Med. 2023;48:289–95. https://doi.org/10.1097/rlu.0000000000004566.
Wang G, Hong H, Zang J, Liu Q, Jiang Y, Fan X, et al. Head-to-head comparison of [68Ga]Ga-P16-093 and [68Ga]Ga-PSMA-617 in dynamic PET/CT evaluation of the same group of recurrent prostate cancer patients. Eur J Nucl Med Mol Imaging. 2022;49:1052–62. https://doi.org/10.1007/s00259-021-05539-1.
Green MA, Hutchins GD, Bahler CD, Tann M, Mathias CJ, Territo W, et al. [68Ga]Ga-P16-093 as a PSMA-targeted PET radiopharmaceutical for detection of cancer: initial evaluation and comparison with [68Ga]Ga-PSMA-11 in prostate cancer patients presenting with biochemical recurrence. Mol Imaging Biol. 2020;22:752–63. https://doi.org/10.1007/s11307-019-01421-7.
Sanchez-Crespo A. Comparison of gallium-68 and fluorine-18 imaging characteristics in positron emission tomography. Appl Radiat Isot. 2013;76:55–62. https://doi.org/10.1016/j.apradiso.2012.06.034.
Zha Z, Choi SR, Ploessl K, Alexoff D, Zhao R, Zhu L, et al. Radiolabeling optimization and preclinical evaluation of the new PSMA imaging agent [(18)F]AlF-P16-093. Bioconjug Chem. 2021;32:1017–26. https://doi.org/10.1021/acs.bioconjchem.1c00177.
Zhao R, Ke M, Lv J, Liu S, Liu Y, Zhang J, et al. First-in-human study of PSMA-targeting agent, [18F]AlF-P16-093: dosimetry and initial evaluation in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2024. https://doi.org/10.1007/s00259-024-06596-y.
Ye Q, Zeng H, Zhao Y, Zhang W, Dong Y, Fan W, et al. Framing protocol optimization in oncological Patlak parametric imaging with uKinetics. EJNMMI Phys. 2023;10:54. https://doi.org/10.1186/s40658-023-00577-0.
Sun T, Wu Y, Wei W, Fu F, Meng N, Chen H, et al. Motion correction and its impact on quantification in dynamic total-body 18F-fluorodeoxyglucose PET. EJNMMI Phys. 2022;9:62. https://doi.org/10.1186/s40658-022-00493-9.
Zanotti-Fregonara P, Chen K, Liow JS, Fujita M, Innis RB. Image-derived input function for brain PET studies: many challenges and few opportunities. J Cerebr Blood F Met. 2011;31:1986–98. https://doi.org/10.1038/jcbfm.2011.107.
van der Weerdt AP, Klein LJ, Boellaard R, Visser CA, Visser FC, Lammertsma AA. Image-derived input functions for determination of MRGlu in cardiac (18)F-FDG PET scans. J Nucl Med. 2001;42:1622–9.
Naganawa M, Gallezot JD, Shah V, Mulnix T, Young C, Dias M, et al. Assessment of population-based input functions for Patlak imaging of whole body dynamic (18)F-FDG PET. EJNMMI Phys. 2020;7:67. https://doi.org/10.1186/s40658-020-00330-x.
Gameraddin M. Normal abdominal aorta diameter on abdominal sonography in healthy asymptomatic adults: impact of age and gender. J Radiat Res Appl Sci. 2019;12:186–91. https://doi.org/10.1080/16878507.2019.1617553.
Turkheimer FE, Hinz R, Cunningham VJ. On the undecidability among kinetic models: from model selection to model averaging. J Cerebr Blood F Met. 2003;23:490–8. https://doi.org/10.1097/01.Wcb.0000050065.57184.Bb.
Haberkorn U, Eder M, Kopka K, Babich JW, Eisenhut M. New strategies in prostate cancer: prostate-specific membrane antigen (PSMA) ligands for diagnosis and therapy. Clin Cancer Res. 2016;22:9–15. https://doi.org/10.1158/1078-0432.Ccr-15-0820.
Łuczyńska E, Anioł J. Neoangiogenesis in prostate cancer. Contemp Oncol (Pozn). 2013;17:229–33. https://doi.org/10.5114/wo.2013.35272.
Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. https://doi.org/10.1038/ng1935.
Minner S, Wittmer C, Graefen M, Salomon G, Steuber T, Haese A, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate. 2011;71:281–8. https://doi.org/10.1002/pros.21241.
van den Hoff J, Oehme L, Schramm G, Maus J, Lougovski A, Petr J, et al. The PET-derived tumor-to-blood standard uptake ratio (SUR) is superior to tumor SUV as a surrogate parameter of the metabolic rate of FDG. EJNMMI Res. 2013;3:77. https://doi.org/10.1186/2191-219x-3-77.
Volpi T, Maccioni L, Colpo M, Debiasi G, Capotosti A, Ciceri T, et al. An update on the use of image-derived input functions for human PET studies: new hopes or old illusions? EJNMMI Res. 2023;13:97. https://doi.org/10.1186/s13550-023-01050-w.
Siebinga H, Heuvel JO, Rijkhorst EJ, Hendrikx J, De Wit-van der Veen BJ. The impact of peptide amount on tumor uptake to assess PSMA receptor saturation on (68)Ga-PSMA-11 PET/CT in patients with primary prostate cancer. J Nucl Med. 2023;64:63–8. https://doi.org/10.2967/jnumed.122.264101.
Acknowledgements
The authors thank Dr. William Eckelman for his advice and suggestions in the preparation of this manuscript.
Funding
This study was supported by Guangdong regional joint fund (2022A1515110941); Guangzhou basic and applied basic research (2023A04J1196), Science and technology program of Guangzhou (202201020558), enhancing scientific research at Guangzhou Medical University, Scientific Instrument Innovation Team of the Chinese Academy of Sciences (GJJSTD20180002), the Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province (2023B1212060052), Shenzhen Science and Technology Innovation Committee (20220531100209020), and the Department of Science and Technology of Guangdong Province (2022A1515110716).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by RZ, ZX, MK, JL, HZ, YH, DG, YL, GZ, LZ, DA, HF.K, XW, and TS. The first draft of the manuscript was written by RZ and ZX, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (ES-2023-141), and it was conducted in accordance with the principles of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all participants included in the study.
Conflict of interest
David Alexoff and Hank F. Kung are employees of Five Eleven Pharma, and Hank Kung is also the founder and board of the company, which holds the patent rights for [18F]AlF-P16-093 and related technology. Other authors have no conflicts of interest or relevant financial activities to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, R., Xia, Z., Ke, M. et al. Determining the optimal pharmacokinetic modelling and simplified quantification method of [18F]AlF-P16-093 for patients with primary prostate cancer (PPCa). Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06624-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06624-x