Abstract
Purpose
Radiation-induced lung injury (RILI) is a severe side effect of radiotherapy (RT) for thoracic malignancies and we currently lack established methods for the early detection of RILI. In this study, we synthesized a new tracer, [18F]AlF-NOTA-QHY-04, targeting C-X-C-chemokine-receptor-type-4 (CXCR4) and investigated its feasibility to detect RILI.
Methods
An RILI rat model was constructed and scanned with [18F]AlF-NOTA-QHY-04 PET/CT and [18F]FDG PET/CT periodically after RT. Dynamic, blocking, autoradiography, and histopathological studies were performed on the day of peak uptake. Fourteen patients with radiation pneumonia, developed during or after thoracic RT, were subjected to PET scan using [18F]AlF-NOTA-QHY-04.
Results
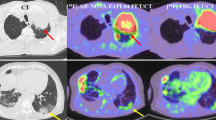
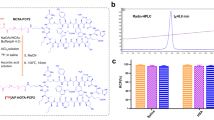
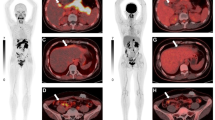
The yield of [18F]AlF-NOTA-QHY-04 was 28.5–43.2%, and the specific activity was 27–33 GBq/μmol. [18F]AlF-NOTA-QHY-04 was mainly excreted through the kidney. Significant increased [18F]AlF-NOTA-QHY-04 uptake in the irradiated lung compared with that in the normal lung in the RILI model was observed on day 6 post-RT and peaked on day 14 post-RT, whereas no apparent uptake of [18F]FDG was shown on days 7 and 15 post-RT. MicroCT imaging did not show pneumonia until 42 days post-RT. Significant intense [18F]AlF-NOTA-QHY-04 uptake was confirmed by autoradiography. Immunofluorescence staining demonstrated expression of CXCR4 was significantly increased in the irradiated lung tissue, which correlated with results obtained from hematoxylin–eosin and Masson’s trichrome staining. In 14 patients with radiation pneumonia, maximum standardized uptake values (SUVmax) were significantly higher in the irradiated lung compared with those in the normal lung. SUVmax of patients with grade 2 RILI was significantly higher than that of patients with grade 1 RILI.
Conclusion
This study indicated that [18F]AlF-NOTA-QHY-04 PET/CT imaging can detect RILI non-invasively and earlier than [18F]FDG PET/CT in a rat model. Clinical studies verified its feasibility, suggesting the clinical potential of [18F]AlF-NOTA-QHY-04 as a PET/CT tracer for early monitoring of RILI.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Yan Y, Fu J, Kowalchuk R, Wright C, Zhang R, Li X, Xu Y. Exploration of radiation-induced lung injury, from mechanism to treatment: a narrative review. Transl Lung Cancer Res. 2022;11(2):307–22. https://doi.org/10.21037/tlcr-22-108.
Giuranno L, Ient J, De Ruysscher D, Vooijs MA. Radiation-induced lung injury (RILI). Front Oncol. 2019;9. https://doi.org/10.3389/fonc.2019.00877.
Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019:156(1). https://doi.org/10.1016/j.chest.2019.03.033.
Désogèr P, Tapias LF, Rietz TA, Rotile N, Blasi F, Day, H, Elliott J, Fuchs BC, Lanuti M, Caravan P. Optimization of a collagen-targeted PET probe for molecular imaging of pulmonary fibrosis. J Nucl Med. 2017. https://doi.org/10.2967/jnumed.117.193532.
Désogèr P, Tapias LF, Hariri LP, Rotile N, Rietz TA, Probst CK, Blasi F, Day H, et al. Type I collagen–targeted PET probe for pulmonary fibrosis detection and staging in preclinical models. Sci Transl Med 2017. https://doi.org/10.1126/scitranslmed.aaf4696.
Saleem A, Helo Y, Win Z, Dale R, Cook J, Searle GE, Wells P. Integrin alpha v beta 6 positron emission tomography imaging in lung cancer patients treated with pulmonary radiation therapy. Int J Radiat Oncol Biol Phys. 2020;2:107. https://doi.org/10.1016/j.ijrobp.2020.02.014.
Qin X, Wang S, Liu X, Duan J, Cheng K, Mu Z, Jia J, Wei Y, Yuan S. Diagnostic value of 18F-NOTA-FAPI PET/CT in a rat model of radiation-induced lung damage. Front Oncol. 2022:12. https://doi.org/10.3389/fonc.2022.879281.
Withana NP, Ma X, Mcguire HM, Verdoes M, Van dL, Wouter A, Ofori LO, Zhang R, Li H, Sanman LE, Wei K. Non-invasive imaging of idiopathic pulmonary fibrosis using cathepsin protease probes. Sci Rep. https://doi.org/10.1038/srep19755
Kircher M, Tran-Gia J, Kemmer L, Zhang X, Schirbel A, Werner RA, Buck AK, Wester H-J, Hacker M, Lapa C, et al. Imaging inflammation in atherosclerosis with CXCR4-directed 68Ga-Pentixafor PET/CT: correlation with 18F-FDG PET/CT. J Nucl Med. 2020;61(5):751–6. https://doi.org/10.2967/jnumed.119.234484.
Hess A, Derlin T, Koenig T, Diekmann J, Wittneben A, Wang Y, Wester H-J, Ross TL, Wollert KC, Bauersachs J, et al. Molecular imaging-guided repair after acute myocardial infarction by targeting the chemokine receptor CXCR4. Eur Heart J. 2020;41(37):3564–75. https://doi.org/10.1093/eurheartj/ehaa598.
Bouter C, Meller B, Sahlmann CO, Staab W, Wester HJ, Kropf S, Meller J. 68Ga-Pentixafor PET/CT imaging of chemokine receptor CXCR4 in chronic infection of the bone: first insights. J Nucl Med. 2018;59(2):320–6. https://doi.org/10.2967/jnumed.117.193854.
Derlin T, Gueler F, Bräsen JH, Schmitz J, Hartung D, Herrmann TR, Ross TL, Wacker F, Wester H-J, Hiss M, et al. Integrating MRI and chemokine receptor CXCR4-targeted PET for detection of leukocyte infiltration in complicated urinary tract infections after kidney transplantation. J Nucl Med. 2017;58(11):1831–7. https://doi.org/10.2967/jnumed.117.193037.
Basuli F, Zhang X, Phelps T, Jagoda E, Choyke P, Swenson R. Automated synthesis of fluorine-18 labeled CXCR4 ligand via the conjugation with nicotinic acid -hydroxysuccinimide ester (6-[18F]SFPy). Molecules. 2020;25(17). https://doi.org/10.3390/molecules25173924.
Luyten K, Van Loy T, Cawthorne C, Deroose C, Schols D, Bormans G, Cleeren F. D-Peptide-based probe for CXCR4-targeted molecular imaging and radionuclide therapy. Pharmaceutics. 2021;13(10). https://doi.org/10.3390/pharmaceutics13101619.
Amor-Coarasa A, Kelly J, Ponnala S, Vedvyas Y, Nikolopoulou A, Williams C, Jin M, David Warren J, Babich J. [F]RPS-544: a PET tracer for imaging the chemokine receptor CXCR4. Nucl Med Biol. 2018;60:37–44. https://doi.org/10.1016/j.nucmedbio.2018.01.004.
Conti M, Eriksson L. Physics of pure and non-pure positron emitters for PET: a review and a discussion. EJNMMI Phys. 2016;3(1):8. https://doi.org/10.1186/s40658-016-0144-5.
Wu F, Zhang Z, Wang M, Ma Y, Verma V, Xiao C, Zhong T, Chen X, Wu M, Yu J, et al. Cellular atlas of senescent lineages in radiation- or immunotherapy-induced lung injury by single-cell RNA-sequencing analysis. Int J Radiat Oncol *Biology* Phys. 2023. https://doi.org/10.1016/j.ijrobp.2023.02.005.
Luo H, Liu X, Liu H, Wang Y, Xu K, Li J, Liu M, Guo J, Qin X. ACT001 Ameliorates ionizing radiation-induced lung injury by inhibiting NLRP3 inflammasome pathway. Biomed Pharmacother. 2023;163:114808. https://doi.org/10.1016/j.biopha.2023.114808.
Curras-Alonso S, Soulier J, Defard T, Weber C, Heinrich S, Laporte H, Leboucher S, Lameiras S, Dutreix M, Favaudon V, et al. An interactive murine single-cell atlas of the lung responses to radiation injury. Nat Commun. 2023;14(1):2445. https://doi.org/10.1038/s41467-023-38134-z.
Feng Y, Yuan P, Guo H, Gu L, Yang Z, Wang J, Zhu W, Zhang Q, Cao J, Wang L, et al. METTL3 mediates epithelial-mesenchymal transition by modulating FOXO1 mRNA N -methyladenosine-dependent YTHDF2 binding: a novel mechanism of radiation-induced lung injury. Adv Sci. 2023:e2204784. https://doi.org/10.1002/advs.202204784.
Cho J, Kodym R, Seliounine S, Richardson JA, Solberg TD, Story M. High dose–per-fraction irradiation of limited lung volumes using an image-guided, highly focused irradiator: simulating stereotactic body radiotherapy regimens in a small-animal model. Int J Radiat Oncol Biol Phys. 2010;77(3):895–902. https://doi.org/10.1016/j.ijrobp.2009.12.074.
Wei Y, Sun Y, Liu J, Zhang G, Qin X, Xu S, Wang S, Tao Y, Pei J, Yu J. Early detection of radiation-induced myocardial damage by [18F]AlF-NOTA-FAPI-04 PET/CT imaging. Eur J Nucl Med Mol Imaging. 2022. https://doi.org/10.1007/s00259-022-05962-y.
Xu M, Guo J, Gu J, Zhang L, Liu Z, Ding L, Fu H, Ma Y, Liang S, Wang H. Preclinical and clinical study on [18F]DRKXH1: a novel β-amyloid PET tracer for Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2021;49(2):652–63. https://doi.org/10.1007/s00259-021-05421-0.
Rahi M, Parekh J, Pednekar P, Parmar G, Abraham S, Nasir S, et al. Radiation-induced lung injury-current perspectives and management. Clin Pract. 2021;11(3):410–29. https://doi.org/10.3390/clinpract11030056.
Derlin T, Jaeger B, Jonigk D, Apel R, Freise J, Shin H, Weiberg D, Warnecke G, Ross T, Wester H, et al. Clinical molecular imaging of pulmonary CXCR4 expression to predict outcome of pirfenidone treatment in idiopathic pulmonary fibrosis. Chest. 2021;159(3):1094–106. https://doi.org/10.1016/j.chest.2020.08.2043.
Vorster M. Gallium-68 labelled radiopharmaceuticals for imaging inflammatory disorders. Semin Nucl Med. 2023;53(2):199–212. https://doi.org/10.1053/j.semnuclmed.2022.08.005.
Schottelius M, Herrmann K, Lapa C. In vivo targeting of CXCR4-new horizons. Cancers. 2021;13(23). https://doi.org/10.3390/cancers13235920.
Zhang X, Chen M, Fan P, Su T, Liang S, Jiang W. Prediction of the mechanism of sodium butyrate against radiation-induced lung injury in non-small cell lung cancer based on network pharmacology and molecular dynamic simulations and molecular dynamic simulations. Front Oncol. 2022;12: 809772. https://doi.org/10.3389/fonc.2022.809772.
Berg J, Halvorsen AR, Bengtson M-B, Lindberg M, Halvorsen B, Aukrust P, Helland Å, Ueland T. Circulating T cell activation and exhaustion markers are associated with radiation pneumonitis and poor survival in non-small-cell lung cancer. Front Immunol. 2022;13. https://doi.org/10.3389/fimmu.2022.875152.
Jung W, Shim S, Kim K. CT findings of acute radiation-induced pneumonitis in breast cancer. Br J Radiol. 2021;94(1124):20200997. https://doi.org/10.1259/bjr.20200997.
Zhang F, Liao L, Wei S, Lu Y. Risk factors of acute radiation-induced lung injury by radiotherapy for esophageal cancer. Comput Math Methods Med. 2022. https://doi.org/10.1155/2022/2416196.
Käsmann L, Dietrich A, Staab-Weijnitz C, Manapov F, Behr J, Rimner A, Jeremic B, Senan S, De Ruysscher D, Lauber K, et al. Radiation-induced lung toxicity - cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat Oncol. 2020;15(1):214. https://doi.org/10.1186/s13014-020-01654-9.
Arroyo-Hernández M, Maldonado F, Lozano-Ruiz F, Muñoz-Montaño W, Nuñez-Baez M, Arrieta O. Radiation-induced lung injury: current evidence. BMC Pulm Med. 2021;21(1). https://doi.org/10.1186/s12890-020-01376-4
Dunsmore S, Shapiro S. The bone marrow leaves its scar: new concepts in pulmonary fibrosis. J Clin Investig. 2004;113(2):180–2. https://doi.org/10.1172/JCI20782.
Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113(2):243–52. https://doi.org/10.1172/JCI18847.
Ishii G, Sangai T, Sugiyama K, Ito T, Hasebe T, Endoh Y, Magae J, Ochiai A. In vivo characterization of bone marrow-derived fibroblasts recruited into fibrotic lesions. Stem cells (Dayton, Ohio). 2005;23(5):699–706. https://doi.org/10.1634/stemcells.2004-0183.
Zhang C, Zhu Y, Wang J, Hou L, Li W, An H. CXCR4-overexpressing umbilical cord mesenchymal stem cells enhance protection against radiation-induced lung injury. Stem Cells Int. 2019;2019:1–12. https://doi.org/10.1155/2019/2457082.
Shi W, Shu H-KG, Yoon Y, Hong S, Xu K, Gao H, Hao C, Torres-Gonzalez E, Nayra C, Rojas M, et al. Inhibition of the CXCL12/CXCR4-axis as preventive therapy for radiation-induced pulmonary fibrosis. PLoS ONE. 2013;8(11):e79768. https://doi.org/10.1371/journal.pone.0079768.
Cao Q, Huang C, Yi H, Gill A, Chou A, Foley M, Hosking C, Lim K, Triffon C, Shi Y, et al. A single-domain i-body, AD-114, attenuates renal fibrosis through blockade of CXCR4. JCI Insight. 2022;7(4). https://doi.org/10.1172/jci.insight.143018.
Zhang W, Zhang R, Ge Y, Wang D, Hu Y, Qin X, Kan J, Liu Y. S100a16 deficiency prevents hepatic stellate cells activation and liver fibrosis via inhibiting CXCR4 expression. Metabolism. 2022;135:155271. https://doi.org/10.1016/j.metabol.2022.155271.
Song H, Yu J, Kong F, Lu J, Bai T, Ma L, Fu Z. [18F]2-Fluoro-2-deoxyglucose positron emission tomography/computed tomography in predicting radiation pneumonitis. Chin Med J. 2009;122(11):1311–5. https://doi.org/10.3760/cma.j.issn.0366-6999.2009.11.014.
Pegah J. Kamyar, Pournazari, Drew, Torigian, Thomas, Werner: A prospective study of the feasibility of FDG-PET/CT imaging to quantify radiation-induced lung inflammation in locally advanced non-small cell lung cancer patients receiving proton or photon radiotherapy. Eur J Nucl Med Mol Imaging. 2018. https://doi.org/10.1007/s00259-018-4154-5.
Ahn H, Kim JH, Lee KC, Park JA, Kim JY, Lee YJ, Lee YJ. Early prediction of radiation-induced pulmonary fibrosis using gastrin-releasing peptide receptor-targeted PET imaging. Mol Pharm. 2023;20(1):267–78. https://doi.org/10.1021/acs.molpharmaceut.2c00632.
Bernchou U, Schytte T, Bertelsen A, Bentzen SM, Hansen O, Brink C. Time evolution of regional CT density changes in normal lung after IMRT for NSCLC. Radiother Oncol. 2013;109(1):89–94. https://doi.org/10.1016/j.radonc.2013.08.041.
Begosh-Mayne D, Kumar SS, Toffel S, Okunieff P, O’Dell W. The dose–response characteristics of four NTCP models: using a novel CT-based radiomic method to quantify radiation-induced lung density changes. Sci Rep. 2020;10(1). https://doi.org/10.1038/s41598-020-67499-0.
Gassert F, Burkhardt R, Gora T, Pfeiffer D, Fingerle A, Sauter A, Schilling D, Rummeny E, Schmid T, Combs S, et al. X-ray dark-field CT for early detection of radiation-induced lung injury in a murine model. Radiology. 2022;303(3):696–8. https://doi.org/10.1148/radiol.212332.
Jin H, Jeon S, Kang G, Lee H, Cho J, Lee Y. Identification of radiation response genes and proteins from mouse pulmonary tissues after high-dose per fraction irradiation of limited lung volumes. Int J Radiat Biol. 2017;93(2):184–93. https://doi.org/10.1080/09553002.2017.1235297.
Hong Z, Eun S, Park K, Choi W, Lee J, Lee E, Lee J, Story M, Cho J. Development of a small animal model to simulate clinical stereotactic body radiotherapy-induced central and peripheral lung injuries. J Radiat Res. 2014;55(4):648–57. https://doi.org/10.1093/jrr/rrt234.
Funding
The study was supported by funding from the Academic Promotion Program of Shandong First Medical University (2019ZL002), the Research Unit of Radiation Oncology, Chinese Academy of Medical Sciences (2019RU071), the foundation of National Natural Science Foundation of China (81627901, 81972863, 82030082, and 82203014), the foundation of Natural Science Foundation of Shandong (ZR201911040452, ZR2019LZL018, ZR2020QH327, and ZR2022QH017), Taishan Scholars Program (NO.tsqn202306386) and the Youth Foundation Cultivation Project of Shandong First Medical University (202201–113).
Author information
Authors and Affiliations
Contributions
Conception and design: JY and JL.
Acquiring data: JP, KC, TL, SW, YG, and BW.
Analyzing data: MG, SX, LM, and WL.
Drafting manuscript: JP and JL.
Revising the manuscript: JY and JL.
Approving the final content of the manuscript: All co-authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Patients gave their oral and written informed consent. This study was approved by the Institutional Review Board of Shandong Cancer Hospital and Institute (approval no. SDTHEC2022011022).
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
259_2023_6517_MOESM1_ESM.png
Supplementary file1 (PNG 466 KB) Supplementary Fig. 1. Dose distributions in the transverse, sagittal, and coronal sections and dose–volume histogram (DVH).
259_2023_6517_MOESM2_ESM.png
Supplementary file2 (PNG 9 KB) Supplementary Fig. 2. The stability of [18F]AlF-NOTA-QHY-04 in serum (Red line for 2h;Black line for 6h).
259_2023_6517_MOESM4_ESM.tif
Supplementary file4 (TIF 18497 KB) Supplementary Fig. 4. Earlier detection of RILI by [18F]AlF-NOTA-QHY-04 PET/CT in comparison with microCT imaging.
259_2023_6517_MOESM5_ESM.tif
Supplementary file5 (TIF 39954 KB) Supplementary Fig. 5. Acute toxicity of [18F]AlF-NOTA-QHY-04. A. Blood biochemistry analysis of mice after treatment with [18F]AlF-NOTA-QHY-04 or PBS; B. Representative H&E staining images of the major organs obtained. ALT: glutamic-pyruvic transminase; AST: glutamic-oxaloacetic transaminase; UA: uric acid; CREA: creatinine.
259_2023_6517_MOESM6_ESM.tif
Supplementary file6 (TIF 13921 KB) Supplementary Fig. 6. Representative [18F]AlF-NOTA-QHY-04 PET/CT images from patient without RILI. A. Baseline lung CT image before radiotherapy; B. Lung CT image after radiotherapy; C. [18F]AlF-NOTA-QHY-04 PET/CT image taken at one month after radiotherapy; D. Dose–volume histogram for patient, showing the radiation dose to the tumor and normal organs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pei, J., Cheng, K., Liu, T. et al. Early, non-invasive detection of radiation-induced lung injury using PET/CT by targeting CXCR4. Eur J Nucl Med Mol Imaging 51, 1109–1120 (2024). https://doi.org/10.1007/s00259-023-06517-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06517-5