Abstract
Purpose
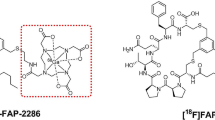
Research on fibroblast activating protein (FAP)-targeting inhibitor (FAPI) has become an important focus for cancer imaging and radiotherapy. Quinoline-based tracers [68 Ga]FAPI-04 and [18F]FAPI-42 have been widely used for positron emission tomography (PET) imaging of most tumors. However, there exist some limitations of these tracers with high uptake in biliary duct system and unstable uptake in pancreas, unsuitable for abdominal tumors PET imaging. Here we developed a [18F]-labeled glycopeptide-containing FAPI tracer (named [18F]FAPT) for PET imaging of FAP in cancers.
Methods
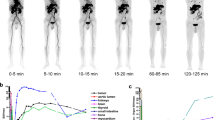
[18F]FAPT was synthesized manually and automatically. The competitive binding to FAP, cellular internalization, and efflux characteristics were examined in vitro using A549-FAP cells. Dynamic MicroPET and biodistribution studies of [18F]FAPT were then conducted in A549-FAP and U87MG xenograft tumor mouse models compared with [18F]FAPI-42. Five healthy volunteers and three patients with cancer underwent [18F]FAPT PET/CT.
Results
Preclinical and clinical studies showed specific binding of [18F]FAPT to FAP and favorable pharmacokinetic properties with better hydrophilicity, lower uptake in biliary duct system, higher tumor uptake and longer tumor retention compared with [18F]FAPI-42. The biodistribution of [18F]FAPT in healthy volunteers and patients with cancer displayed low uptake in most normal tissues except for pancreas, thyroid and salivary gland, which could contribute to high tumor-to-background ratios in most cancers.
Conclusion
[18F]FAPT is better PET tracer than [18F]FAPI-42 for imaging of biliary duct system cancer, potentially providing a tool to examine FAP expression in most cancers with high tumor-to-background ratios.








Similar content being viewed by others
Data availability
The data sets generated and analyzed during this study, if reasonably required, can be obtained from the correspondence author.
References
Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin Appl. 2014;8:454–63. https://doi.org/10.1002/prca.201300095.
Liu R, Li H, Liu L, Yu J, Ren X. Fibroblast activation protein: A potential therapeutic target in cancer. Cancer Biol Ther. 2012;13:123–9. https://doi.org/10.4161/cbt.13.3.18696.
Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA. 1990;87:7235–9. https://doi.org/10.1073/pnas.87.18.7235.
Hu K, Wang L, Wu H, Huang S, Tian Y, Wang Q, et al. [(18)F]FAPI-42 PET imaging in cancer patients: optimal acquisition time, biodistribution, and comparison with [(68)Ga]Ga-FAPI-04. Eur J Nucl Med Mol Imaging. 2022;49:2833–43. https://doi.org/10.1007/s00259-021-05646-z.
Imlimthan S, Moon ES, Rathke H, Afshar-Oromieh A, Rösch F, Rominger A, et al. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals (Basel, Switzerland). 2021;14. https://doi.org/10.3390/ph14101023.
Ballal S, Yadav MP, Moon ES, Kramer VS, Roesch F, Kumari S, et al. Biodistribution, pharmacokinetics, dosimetry of [(68)Ga]Ga-DOTA.SA.FAPi, and the head-to-head comparison with [(18)F]F-FDG PET/CT in patients with various cancers. Eur J Nucl Med Mol Imaging. 2021;48:1915–31. https://doi.org/10.1007/s00259-020-05132-y.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J Nucl Med. 2018;59:1415–22. https://doi.org/10.2967/jnumed.118.210443.
Jansen K, Heirbaut L, Verkerk R, Cheng JD, Joossens J, Cos P, et al. Extended structure-activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J Med Chem. 2014;57:3053–74. https://doi.org/10.1021/jm500031w.
Jansen K, Heirbaut L, Cheng JD, Joossens J, Ryabtsova O, Cos P, et al. Selective Inhibitors of Fibroblast Activation Protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine Scaffold. ACS Med Chem Lett. 2013;4:491–6. https://doi.org/10.1021/ml300410d.
Chen H, Zhao L, Ruan D, Pang Y, Hao B, Dai Y, et al. Usefulness of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [(18)F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging. 2021;48:73–86. https://doi.org/10.1007/s00259-020-04940-6.
Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47:1820–32. https://doi.org/10.1007/s00259-020-04769-z.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med. 2019;60:801–5. https://doi.org/10.2967/jnumed.119.227967.
Zhang X, Song W, Qin C, Liu F, Lan X. Non-malignant findings of focal (68)Ga-FAPI-04 uptake in pancreas. Eur J Nucl Med Mol Imaging. 2021;48:2635–41. https://doi.org/10.1007/s00259-021-05194-6.
Li H, Ye S, Li L, Zhong J, Yan Q, Zhong Y, et al. (18)F- or (177)Lu-labeled bivalent ligand of fibroblast activation protein with high tumor uptake and retention. Eur J Nucl Med Mol Imaging. 2022;49:2705–15. https://doi.org/10.1007/s00259-022-05757-1.
Hu K, Li J, Wang L, Huang Y, Li L, Ye S, et al. Preclinical evaluation and pilot clinical study of [(18)F]AlF-labeled FAPI-tracer for PET imaging of cancer associated fibroblasts. Acta pharmaceutica Sinica B. 2022;12:867–75. https://doi.org/10.1016/j.apsb.2021.09.032.
Wang S, Zhou X, Xu X, Ding J, Liu S, Hou X, et al. Clinical translational evaluation of Al(18)F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur J Nucl Med Mol Imaging. 2021;48:4259–71. https://doi.org/10.1007/s00259-021-05470-5.
Jiang X, Wang X, Shen T, Yao Y, Chen M, Li Z, et al. FAPI-04 PET/CT Using [(18)F]AlF Labeling Strategy: Automatic Synthesis, Quality Control, and In Vivo Assessment in Patient. Front Oncol. 2021;11:649148. https://doi.org/10.3389/fonc.2021.649148.
Giesel FL, Adeberg S, Syed M, Lindner T, Jiménez-Franco LD, Mavriopoulou E, et al. FAPI-74 PET/CT Using Either (18)F-AlF or Cold-Kit (68)Ga Labeling: Biodistribution, Radiation Dosimetry, and Tumor Delineation in Lung Cancer Patients. J Nucl Med. 2021;62:201–7. https://doi.org/10.2967/jnumed.120.245084.
Toms J, Kogler J, Maschauer S, Daniel C, Schmidkonz C, Kuwert T, et al. Targeting Fibroblast Activation Protein: Radiosynthesis and Preclinical Evaluation of an (18)F-Labeled FAP Inhibitor. J Nucl Med. 2020;61:1806–13. https://doi.org/10.2967/jnumed.120.242958.
Huang J, Fu L, Hu K, Huang S, Han Y, Lin R, et al. Automatic Production and Preliminary PET Imaging of a New Imaging Agent [(18)F]AlF-FAPT. Front Oncol. 2021;11:802676. https://doi.org/10.3389/fonc.2021.802676.
Schottelius M, Wester HJ, Reubi JC, Senekowitsch-Schmidtke R, Schwaiger M. Improvement of pharmacokinetics of radioiodinated Tyr(3)-octreotide by conjugation with carbohydrates. Bioconjug Chem. 2002;13:1021–30. https://doi.org/10.1021/bc0200069.
Niedermoser S, Chin J, Wängler C, Kostikov A, Bernard-Gauthier V, Vogler N, et al. In Vivo Evaluation of 18F-SiFAlin-Modified TATE: A Potential Challenge for 68Ga-DOTATATE, the Clinical Gold Standard for Somatostatin Receptor Imaging with PET. J Nucl Med. 2015;56:1100–5. https://doi.org/10.2967/jnumed.114.149583.
Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, et al. Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Can Res. 2001;61:1781–5.
Xu M, Zhang P, Ding J, Chen J, Huo L, Liu Z. Albumin Binder-Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy. J Nucl Med. 2022;63:952–8. https://doi.org/10.2967/jnumed.121.262533.
Tran E, Chinnasamy D, Yu Z, Morgan RA, Lee CC, Restifo NP, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210:1125–35. https://doi.org/10.1084/jem.20130110.
Acknowledgements
We thank our colleagues in the Nanfang PET center who manufactured the radiopharmaceuticals and performed the microPET and PET/CT examination. We sincerely thank the Guangzhou Atom High Tech Radiopharmaceutical Co., Ltd for giving us help in radionuclide drugs.
Funding
This work is supported by the Guangdong Basic and Applied Basic Research Foundation (2022A1515010072, 2022A1515110051, 2020A1515011399), Guangzhou Science and Technology Plan (2023B03J0529), Medical Products Administration of Guangdong Province (2021ZDB02, Drug Supervision and Administration Division 1 (2022)), Nanfang Hospital Talent Introduction Fundation of Southern Medical University (123456), and the National Natural Science Foundation of China (91949121).
Author information
Authors and Affiliations
Contributions
Study conception and design, GT. Acquiring data, JH, LF, XZ, DY, KH, SH, RL, and YJH. Analysis of data, JH, LF, KH, and SH. Drafting the manuscript, JH and LF. Revising the manuscript, GT. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Chinese Ethics Committee of Registering Clinical Trials (ChiECRCT20210617) and registered on the Chinese Clinical Trial Registry (ChiCTR2200059004).
Consent to participate
Informed consents were obtained from all individual participants included in the study.
Consent for publication
All the authors approved the publication of this article.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, J., Fu, L., Zhang, X. et al. Noninvasive imaging of FAP expression using positron emission tomography: A comparative evaluation of a [18F]-labeled glycopeptide-containing FAPI with [18F]FAPI-42. Eur J Nucl Med Mol Imaging 50, 3363–3374 (2023). https://doi.org/10.1007/s00259-023-06282-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06282-5