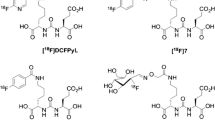
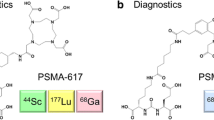
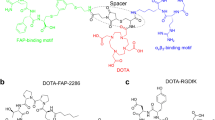
Abstract
Purpose
We developed a theranostic radiopharmaceutical that engages two key cell surface proteases, fibroblast activation protein alpha (FAP) and prostate-specific membrane antigen (PSMA), each frequently overexpressed within the tumor microenvironment (TME). The latter is also expressed in most prostate tumor epithelium. To engage a broader spectrum of cancers for imaging and therapy, we conjugated small-molecule FAP and PSMA-targeting moieties using an optimized linker to provide 64Cu-labeled compounds.
Methods
We synthesized FP-L1 and FP-L2 using two linker constructs attaching the FAP and PSMA-binding pharmacophores. We determined in vitro inhibition constants (Ki) for FAP and PSMA. Cell uptake assays and flow cytometry were conducted in human glioma (U87), melanoma (SK-MEL-24), prostate cancer (PSMA + PC3 PIP and PSMA − PC3 flu), and clear cell renal cell carcinoma lines (PSMA + /PSMA − 786-O). Quantitative positron emission tomography/computed tomography (PET/CT) and tissue biodistribution studies were performed using U87, SK-MEL-24, PSMA + PC3 PIP, and PSMA + 786-O experimental xenograft models and the KPC genetically engineered mouse model of pancreatic cancer.
Results
64Cu-FP-L1 and 64Cu-FP-L2 were produced in high radiochemical yields (> 98%) and molar activities (> 19 MBq/nmol). Ki values were in the nanomolar range for both FAP and PSMA. PET imaging and biodistribution studies revealed high and specific targeting of 64Cu-FP-L1 and 64Cu-FP-L2 for FAP and PSMA. 64Cu-FP-L1 displayed more favorable pharmacokinetics than 64Cu-FP-L2. In the U87 tumor model at 2 h post-injection, tumor uptake of 64Cu-FP-L1 (10.83 ± 1.02%ID/g) was comparable to 64Cu-FAPI-04 (9.53 ± 2.55%ID/g). 64Cu-FP-L1 demonstrated high retention 5.34 ± 0.29%ID/g at 48 h in U87 tumor. Additionally, 64Cu-FP-L1 showed high retention in PSMA + PC3 PIP tumor (12.06 ± 0.78%ID/g at 2 h and 10.51 ± 1.82%ID/g at 24 h).
Conclusions
64Cu-FP-L1 demonstrated high and specific tumor targeting of FAP and PSMA. This compound should enable imaging of lesions expressing FAP, PSMA, or both on the tumor cell surface or within the TME. FP-L1 can readily be converted into a theranostic for the management of heterogeneous tumors.
Graphical abstract








Similar content being viewed by others
Change history
31 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00259-022-05951-1
References
Herrmann K, Schwaiger M, Lewis JS, Solomon SB, McNeil BJ, Baumann M, et al. Radiotheranostics: a roadmap for future development. Lancet Oncol. 2020;21:e146–56. https://doi.org/10.1016/S1470-2045(19)30821-6.
Siva S, Udovicich C, Tran B, Zargar H, Murphy DG, Hofman MS. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat Rev Urol. 2020;17:107–18. https://doi.org/10.1038/s41585-019-0272-5.
Imlimthan S, Moon ES, Rathke H, Afshar-Oromieh A, Rösch F, Rominger A, et al. New frontiers in cancer imaging and therapy based on radiolabeled fibroblast activation protein inhibitors: A rational review and current progress. Pharmaceuticals. 2021;14:1023.
Uijen MJM, Derks YHW, Merkx RIJ, Schilham MGM, Roosen J, Privé BM, et al. PSMA radioligand therapy for solid tumors other than prostate cancer: background, opportunities, challenges, and first clinical reports. Eur J Nucl Med Mol Imaging. 2021;48:4350–68. https://doi.org/10.1007/s00259-021-05433-w.
Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–59. https://doi.org/10.1158/2159-8290.cd-20-1808.
Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA. 1990;87:7235–9. https://doi.org/10.1073/pnas.87.18.7235.
Chang SS, Reuter VE, Heston WDW, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192.
Wernicke AG, Kim S, Liu H, Bander NH, Pirog EC. Prostate-specific membrane antigen (PSMA) expression in the neovasculature of gynecologic malignancies: implications for PSMA-targeted therapy. App Immunohistochem Mol Morphol. 2017;25:271–6. https://doi.org/10.1097/pai.0000000000000297.
Spatz S, Tolkach Y, Jung K, Stephan C, Busch J, Ralla B, et al. Comprehensive evaluation of prostate specific membrane antigen expression in the vasculature of renal tumors: implications for imaging studies and prognostic role. J Urol. 2018;199:370–7. https://doi.org/10.1016/j.juro.2017.08.079.
Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37:154–8. https://doi.org/10.1097/MPA.0b013e31816618ce.
Busek P, Balaziova E, Matrasova I, Hilser M, Tomas R, Syrucek M, et al. Fibroblast activation protein alpha is expressed by transformed and stromal cells and is associated with mesenchymal features in glioblastoma. Tumor Biol. 2016;37:13961–71. https://doi.org/10.1007/s13277-016-5274-9.
López JI, Errarte P, Erramuzpe A, Guarch R, Cortés JM, Angulo JC, et al. Fibroblast activation protein predicts prognosis in clear cell renal cell carcinoma. Human Pathol. 2016;54:100–5. https://doi.org/10.1016/j.humpath.2016.03.009.
Solano-Iturri JD, Beitia M, Errarte P, Calvete-Candenas J, Etxezarraga MC, Loizate A, et al. Altered expression of fibroblast activation protein-α; (FAP) in colorectal adenoma-carcinoma sequence and in lymph node and liver metastases. Aging. 2020;12:10337–58. https://doi.org/10.18632/aging.103261.
Solano-Iturri JD, Errarte P, Etxezarraga MC, Echevarria E, Angulo J, López JI, et al. Altered tissue and plasma levels of fibroblast activation protein-α (FAP) in renal tumours. Cancers. 2020;12:3393.
Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. https://doi.org/10.1016/S0140-6736(21)00237-3.
Paschalis A, Sheehan B, Riisnaes R, Rodrigues DN, Gurel B, Bertan C, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019;76:469–78. https://doi.org/10.1016/j.eururo.2019.06.030.
Hintz HM, Gallant JP, Vander Griend DJ, Coleman IM, Nelson PS, LeBeau AM. Imaging fibroblast activation protein alpha improves diagnosis of metastatic prostate cancer with positron emission tomography. Clin Cancer Res. 2020;26:4882–91. https://doi.org/10.1158/1078-0432.ccr-20-1358.
Kesch C, Yirga L, Dendl K, Handke A, Darr C, Krafft U, et al. High fibroblast-activation-protein expression in castration-resistant prostate cancer supports the use of FAPI-molecular theranostics. Eur J Nucl Med Mol Imaging. 2021;49:385–9. https://doi.org/10.1007/s00259-021-05423-y.
Isik EG, Has-Simsek D, Sanli O, Sanli Y, Kuyumcu S. Fibroblast activation protein–targeted pet imaging of metastatic castration-resistant prostate cancer compared with 68Ga-PSMA and 18F-FDG PET/CT. Clin Nucl Med. 2021. https://doi.org/10.1097/rlu.0000000000003837.
Kessel K, Seifert R, Weckesser M, Boegemann M, Huss S, Kratochwil C, et al. Prostate-specific membrane antigen and fibroblast activation protein distribution in prostate cancer: preliminary data on immunohistochemistry and PET imaging. Ann Nucl Med. 2021. https://doi.org/10.1007/s12149-021-01702-8.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–5. https://doi.org/10.2967/jnumed.119.227967.
Mona CE, Benz MR, Hikmat F, Grogan TR, Lückerath K, Razmaria A, et al. Correlation of 68Ga-FAPi-46 PET biodistribution with FAP expression by immunohistochemistry in patients with solid cancers: a prospective translational exploratory study. J Nucl Med. 2021:jnumed.121.262426. https://doi.org/10.2967/jnumed.121.262426.
Kalluri R. The biology and function of fibroblasts in cancer. Nature Rev Cancer. 2016;16:582–98. https://doi.org/10.1038/nrc.2016.73.
Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Met Rev. 2020;39:783–803. https://doi.org/10.1007/s10555-020-09909-3.
Keane FM, Yao T-W, Seelk S, Gall MG, Chowdhury S, Poplawski SE, et al. Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs. FEBS Open Bio. 2014;4:43–54. https://doi.org/10.1016/j.fob.2013.12.001.
Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein–expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11:257–66. https://doi.org/10.1158/1535-7163.mct-11-0340.
Loktev A, Lindner T, Burger E-M, Altmann A, Giesel F, Kratochwil C, et al. Development of fibroblast activation protein–targeted radiotracers with improved tumor retention. J Nucl Med. 2019;60:1421–9. https://doi.org/10.2967/jnumed.118.224469.
Conway RE, Joiner K, Patterson A, Bourgeois D, Rampp R, Hannah BC, et al. Prostate specific membrane antigen produces pro-angiogenic laminin peptides downstream of matrix metalloprotease-2. Angiogenesis. 2013;16:847–60. https://doi.org/10.1007/s10456-013-9360-y.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–22. https://doi.org/10.2967/jnumed.118.210443.
Ballal S, Yadav MP, Kramer V, Moon ES, Roesch F, Tripathi M, et al. A theranostic approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: new frontier in targeted radionuclide therapy. Eur J Nucl Med Mol Imaging. 2021;48:942–4. https://doi.org/10.1007/s00259-020-04990-w.
Kratochwil C, Giesel FL, Rathke H, Fink R, Dendl K, Debus J, et al. [153Sm]Samarium-labeled FAPI-46 radioligand therapy in a patient with lung metastases of a sarcoma. European J Nucl Med Mol Imaging. 2021;48:3011–3. https://doi.org/10.1007/s00259-021-05273-8.
Assadi M, Jokar N, Ghasemi M, Nabipour I, Gholamrezanezhad A, Ahmadzadehfar H. Precision medicine approach in prostate cancer. Current Pharm Design. 2020;26:3783–98. https://doi.org/10.2174/1381612826666200218104921.
Baum RP, Schuchardt C, Singh A, Chantadisai M, Robiller FC, Zhang J, et al. Feasibility, biodistribution and preliminary dosimetry in peptide-targeted radionuclide therapy (PTRT) of diverse adenocarcinomas using 177Lu-FAP-2286: first-in-human results. J Nucl Med. 2021:jnumed.120.259192. https://doi.org/10.2967/jnumed.120.259192.
Xu M, Zhang P, Ding J, Chen J, Huo L, Liu Z. Albumin binder–conjugated fibroblast activation protein inhibitor radiopharmaceuticals for cancer therapy. J Nucl Med. 2022;63:952–8. https://doi.org/10.2967/jnumed.121.262533.
Moon ES, Ballal S, Yadav MP, Bal C, Van Rymenant Y, Stephan S, et al. Fibroblast activation protein (FAP) targeting homodimeric FAP inhibitor radiotheranostics: a step to improve tumor uptake and retention time. Am J Nucl Med Mol Imaging. 2021;11:476–91.
Li H, Ye S, Li L, Zhong J, Yan Q, Zhong Y, et al. 18F- or 177Lu-labeled bivalent ligand of fibroblast activation protein with high tumor uptake and retention. Eur J Nucl Med Mol Imaging. 2022. https://doi.org/10.1007/s00259-022-05757-1.
Zhao L, Niu B, Fang J, Pang Y, Li S, Xie C, et al. Synthesis, preclinical evaluation, and a pilot clinical pet imaging study of 68Ga-Labeled FAPI dimer. J Nucl Med. 2022;63:862–8. https://doi.org/10.2967/jnumed.121.263016.
Galbiati A, Zana A, Bocci M, Millul J, Elsayed A, Mock J, et al. A novel dimeric FAP-targeting small molecule-radio conjugate with high and prolonged tumour uptake. J Nucl Med. 2022:jnumed.122.264036. https://doi.org/10.2967/jnumed.122.264036.
Röhrich M, Loktev A, Wefers AK, Altmann A, Paech D, Adeberg S, et al. IDH-wildtype glioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein–specific PET/CT. Eur J Nucl Med Mol Imaging. 2019;46:2569–80. https://doi.org/10.1007/s00259-019-04444-y.
Jansen K, Heirbaut L, Verkerk R, Cheng JD, Joossens J, Cos P, et al. Extended structure–activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J Med Chem. 2014;57:3053–74. https://doi.org/10.1021/jm500031w.
Banerjee SR, Pullambhatla M, Byun Y, Nimmagadda S, Foss CA, Green G, et al. Sequential SPECT and optical imaging of experimental models of prostate cancer with a dual-modality inhibitor of the prostate-specific membrane antigen. Angew Chem Int Ed. 2011;50:9167–70. https://doi.org/10.1002/anie.201102872.
Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. https://doi.org/10.1016/0006-2952(73)90196-2.
Banerjee SR, Kumar V, Lisok A, Chen J, Minn I, Brummet M, et al. 177Lu-labeled low-molecular-weight agents for PSMA-targeted radiopharmaceutical therapy. Eur J Nucl Med Mol Imaging. 2019;46:2545–57. https://doi.org/10.1007/s00259-019-04434-0.
Nimmagadda S, Pullambhatla M, Chen Y, Parsana P, Lisok A, Chatterjee S, et al. Low-level endogenous PSMA expression in nonprostatic tumor xenografts is sufficient for in vivo tumor targeting and imaging. J Nucl Med. 2018;59:486–93. https://doi.org/10.2967/jnumed.117.191221.
Olszewski RT, Bukhari N, Zhou J, Kozikowski AP, Wroblewski JT, Shamimi-Noori S, et al. NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J Neurochem. 2004;89:876–85. https://doi.org/10.1111/j.1471-4159.2004.02358.x.
He M, Henderson M, Muth S, Murphy A, Zheng L. Preclinical mouse models for immunotherapeutic and non-immunotherapeutic drug development for pancreatic ductal adenocarcinoma. Ann Pancreat Cancer. 2020;3.
Slania SL, Das D, Lisok A, Du Y, Jiang Z, Mease RC, et al. Imaging of fibroblast activation protein in cancer xenografts using novel (4-quinolinoyl)-glycyl-2-cyanopyrrolidine-based small molecules. J Med Chem. 2021;64:4059–70. https://doi.org/10.1021/acs.jmedchem.0c02171.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Errarte P, Guarch R, Pulido R, Blanco L, Nunes-Xavier CE, Beitia M, et al. The expression of fibroblast activation protein in clear cell renal cell carcinomas is associated with synchronous lymph node metastases. PLoS One. 2016;11: e0169105. https://doi.org/10.1371/journal.pone.0169105.
Meyer AR, Carducci MA, Denmeade SR, Markowski MC, Pomper MG, Pierorazio PM, et al. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med. 2019;33:617–23. https://doi.org/10.1007/s12149-019-01371-8.
Delgado-Bellido D, Serrano-Saenz S, Fernández-Cortés M, Oliver FJ. Vasculogenic mimicry signaling revisited: focus on non-vascular VE-cadherin. Mol Cancer. 2017;16:65. https://doi.org/10.1186/s12943-017-0631-x.
Zhou L, Chang Y, Xu L, Liu Z, Fu Q, Yang Y, et al. The presence of vascular mimicry predicts high risk of clear cell renal cell carcinoma after radical nephrectomy. J Urol. 2016;196:335–42. https://doi.org/10.1016/j.juro.2016.02.2971.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Eng J Med. 2019;380:1116–27. https://doi.org/10.1056/NEJMoa1816714.
Puré E, Blomberg R. Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene. 2018;37:4343–57. https://doi.org/10.1038/s41388-018-0275-3.
Stock K, Steinestel K, Wiesch R, Mikesch J-H, Hansmeier A, Trautmann M, et al. Neovascular prostate-specific membrane antigen expression is associated with improved overall survival under palliative chemotherapy in patients with pancreatic ductal adenocarcinoma. BioMed Res Int. 2017;2017:2847303. https://doi.org/10.1155/2017/2847303.
Pereira BA, Vennin C, Papanicolaou M, Chambers CR, Herrmann D, Morton JP, et al. CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. trends in cancer. 2019;5:724–41. https://doi.org/10.1016/j.trecan.2019.09.010.
Poels TT, Vuijk FA, de Geus-Oei L-F, Vahrmeijer AL, Oprea-Lager DE, Swijnenburg R-J. Molecular targeted positron emission tomography imaging and radionuclide therapy of pancreatic ductal adenocarcinoma. Cancers. 2021;13:6164.
Krishnaraju VS, Kumar R, Mittal BR, Sharma V, Singh H, Nada R, et al. Differentiating benign and malignant pancreatic masses: Ga-68 PSMA PET/CT as a new diagnostic avenue. Euro Radiol. 2021;31:2199–208. https://doi.org/10.1007/s00330-020-07318-2.
Sheridan C. Amgen’s bispecific antibody puffs across finish line. Nat Biotechnol. 2015;33:219–21. https://doi.org/10.1038/nbt0315-219.
Luo H, Hernandez R, Hong H, Graves SA, Yang Y, England CG, et al. Noninvasive brain cancer imaging with a bispecific antibody fragment, generated via click chemistry. Proc Natl Acad Sci. 2015;112:12806–11. https://doi.org/10.1073/pnas.1509667112.
Shallal HM, Minn I, Banerjee SR, Lisok A, Mease RC, Pomper MG. Heterobivalent agents targeting PSMA and integrin-αvβ3. Bioconjug Chem. 2014;25:393–405. https://doi.org/10.1021/bc4005377.
Bandari RP, Carmack TL, Malhotra A, Watkinson L, Fergason Cantrell EA, Lewis MR, et al. Development of heterobivalent theranostic probes having high affinity/selectivity for the GRPR/PSMA. J Med Chem. 2021;64:2151–66. https://doi.org/10.1021/acs.jmedchem.0c01785.
Liolios C, Patsis C, Lambrinidis G, Tzortzini E, Roscher M, Bauder-Wüst U, et al. Investigation of tumor cells and receptor-ligand simulation models for the development of PET imaging probes targeting PSMA and GRPR and a possible crosstalk between the two receptors. Mol Pharm. 2022. https://doi.org/10.1021/acs.molpharmaceut.2c00070.
Watabe T, Liu Y, Kaneda-Nakashima K, Shirakami Y, Lindner T, Ooe K, et al. Theranostics targeting fibroblast activation protein in the tumor stroma: 64Cu- and 225Ac-labeled FAPI-04 in pancreatic cancer xenograft mouse models. J Nucl Med. 2020;61:563–9. https://doi.org/10.2967/jnumed.119.233122.
Funding
We thank Precision Molecular Inc., the Emerson Collective Cancer Research Fund, W81XWH2110920, EB024495, and CA184228 for financial support.
Author information
Authors and Affiliations
Contributions
Sangeeta Ray Banerjee and Martin G. Pomper contributed to the study’s conception and design. Material preparation (Srikanth Boinapally and Sangeeta Ray Banerjee), data collection, and analysis were performed by Srikanth Boinapally, Ala Lisok, Gabriela Lofland, Il Minn, Yu Yan, Zirui Jiang, Min Jay Shin, Vanessa Merino, Cory Brayton, and Sangeeta Ray Banerjee. Sangeeta Ray Banerjee wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All animal studies complied with the regulations of the Johns Hopkins University animal care and use committee.
Competing interests
Under a license agreement with Johns Hopkins University. S.B., I.M., M.G.P., and S.R.B. are entitled to royalty distributions related to the technology described in the study discussed in this publication. This arrangement has been reviewed and approved by Johns Hopkins University following its conflict-of-interest policies.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging.
The original online version of this article was revised: The authors regret that the supplementary file that was captured in their article is incomplete. It is now replaced with the correct version.
The original article has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1
(PDF 2.79 MB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boinapally, S., Lisok, A., Lofland, G. et al. Hetero-bivalent agents targeting FAP and PSMA. Eur J Nucl Med Mol Imaging 49, 4369–4381 (2022). https://doi.org/10.1007/s00259-022-05933-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05933-3