Abstract
Purpose
Dosimetry is critical to achieve the optimal therapeutic effect of radioligand therapy (RLT) with limited side effects. Our aim was to perform image-based absorbed dose calculation for the new PSMA ligand 177Lu-DKFZ-PSMA-617 in support of its use for the treatment of metastatic prostate cancer.
Methods
Whole-body planar images and SPECT/CT images of the abdomen were acquired in five patients (mean age 68 years) for during two treatment cycles at approximately 1, 24, 48 and 72 h after administration of 3.6 GBq (range 3.4 to 3.9 GBq) 177Lu-DKFZ-PSMA-617. Quantitative 3D SPECT OSEM reconstruction was performed with corrections for photon scatter, photon attenuation and detector blurring. A camera-specific calibration factor derived from phantom measurements was used for quantitation. Absorbed doses were calculated for various organs from the images using a combination of linear approximation, exponential fit, and target-specific S values, in accordance with the MIRD scheme. Absorbed doses to bone marrow were estimated from planar and SPECT images and with consideration of the blood sampling method according to the EANM guidelines.
Results
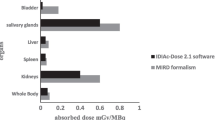
The average (± SD) absorbed doses per cycle were 2.2 ± 0.6 Gy for the kidneys (0.6 Gy/GBq), 5.1 ± 1.8 Gy for the salivary glands (1.4 Gy/GBq), 0.4 ± 0.2 Gy for the liver (0.1 Gy/GBq), 0.4 ± 0.1 Gy for the spleen (0.1 Gy/GBq), and 44 ± 19 mGy for the bone marrow (0.012 Gy/GBq). The organ absorbed doses did not differ significantly between cycles. The critical absorbed dose reported for the kidneys (23 Gy) was not reached in any patient. At 24 h there was increased uptake in the colon with 50 – 70 % overlap to the kidneys on planar images. Absorbed doses for tumour lesions ranged between 1.2 and 47.5 Gy (13.1 Gy/GBq) per cycle.
Conclusion
The salivary glands and kidneys showed high, but not critical, absorbed doses after RLT with 177Lu-DKFZ-PSMA-617. We suggest that 177Lu-DKFZ-PSMA-617 is suitable for radiotherapy, offering tumour-to-kidney ratios comparable to those with RLT agents currently available for the treatment of neuroendocrine tumours. Our dosimetry results suggest that 177Lu-DKFZ-PSMA-617 treatment with higher activities and more cycles is possible without the risk of damaging the kidneys.





Similar content being viewed by others
References
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128.
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Pond GR, Sonpavde G, de Wit R, Eisenberger MA, Tannock IF, Armstrong AJ. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:3–6.
Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–39.
Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, et al. Radiation dosimetry and first therapy results with a (124)I/(131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–92.
Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19:5182–91.
Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004;22:2522–31.
Benesova M, Schafer M, Bauder-Wust U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–20.
Delker A, Ilhan H, Zach C, Brosch J, Gildehaus F-J, Lehner S, et al. The influence of early measurements onto the estimated kidney dose in 177Lu-DOTATATE peptide receptor radiotherapy of neuroendocrine tumors. Mol Imaging Biol. 2015. doi:10.1007/s11307-015-0839-3.
Kohli V, King MA, Glick SJ, Pan TS. Comparison of frequency-distance relationship and gaussian-diffusion-based methods of compensation for distance-dependent spatial resolution in SPECT imaging. Phys Med Biol. 1998;43:1025–37.
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13:601–9.
Wallis JW, Miller TR. An optimal rotator for iterative reconstruction. IEEE Trans Med Imaging. 1997;16:118–23.
Green PJ. Bayesian reconstructions from emission tomography data using a modified EM algorithm. IEEE Trans Med Imaging. 1990;9:84–93.
Beekman FJ, Kamphuis C, Frey EC. Scatter compensation methods in 3D iterative SPECT reconstruction: a simulation study. Phys Med Biol. 1997;42:1619–32.
Bai CY, Shao L, Da Silva AJ, Zhao Z. A generalized model for the conversion from CT numbers to linear attenuation coefficients. IEEE Trans Nucl Sci. 2003;50:1510–5.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3] octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30.
Stabin MS, Hunt, J, Brill, A, Sparks R, Eckerman K, Bertelli L. RADAR: the radiation dose assessment resource. www.doseinfo-radar.com. 2001.
Stabin MG, Siegel JA. Physical models and dose factors for use in internal dose assessment. Health Phys. 2003;85:294–310.
Williams LE, Liu A, Yamauchi DM, Lopatin G, Raubitschek AA, Wong JY. The two types of correction of absorbed dose estimates for internal emitters. Cancer. 2002;94:1231–4.
Hindorf C, Glatting G, Chiesa C, Linden O, Flux G; EANM Dosimetry Committee. EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry. Eur J Nucl Med Mol Imaging. 2010;37:1238–50.
Sgouros G. Bone marrow dosimetry for radioimmunotherapy: theoretical considerations. J Nucl Med. 1993;34:689–94.
Traino AC, Ferrari M, Cremonesi M, Stabin MG. Influence of total-body mass on the scaling of S-factors for patient-specific, blood-based red-marrow dosimetry. Phys Med Biol. 2007;52:5231–48.
Dewaraja YK, Frey EC, Sgouros G, Brill AB, Roberson P, Zanzonico PB, et al. MIRD pamphlet No. 23: quantitative SPECT for patient-specific 3-dimensional dosimetry in internal radionuclide therapy. J Nucl Med. 2012;53:1310–25.
Sanders J, Kuwert T, Hornegger J, Ritt P. Quantitative SPECT/CT imaging of 177Lu with in vivo validation in patients undergoing peptide receptor radionuclide therapy. Mol Imaging Biol. 2015;17:585–93.
Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart H, Hadaschik B, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95.
Gordon IO, Tretiakova MS, Noffsinger AE, Hart J, Reuter VE, Al-Ahmadie HA. Prostate-specific membrane antigen expression in regeneration and repair. Mod Pathol. 2008;21:1421–7.
Maraj B, Aldersley M, Markham A. Prostate-specific membrane antigen expression in the duodenum: implications in coeliac disease and immunotherapy for prostate cancer. Lancet. 1998;351:1559–60.
Silver DA, Pellicer I, Fair WR, Heston W, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Gensheimer MF, Liao JJ, Garden AS, Laramore GE, Parvathaneni U. Submandibular gland-sparing radiation therapy for locally advanced oropharyngeal squamous cell carcinoma: patterns of failure and xerostomia outcomes. Radiat Oncol. 2014;9:255.
Hey J, Setz J, Gerlach R, Janich M, Hildebrandt G, Vordermark D, et al. Parotid gland-recovery after radiotherapy in the head and neck region – 36 months follow-up of a prospective clinical study. Radiat Oncol. 2011;6:125.
Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–601.
Vallabhajosula S, Kuji I, Hamacher KA, Konishi S, Kostakoglu L, Kothari PA, et al. Pharmacokinetics and biodistribution of 111In-and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J Nucl Med. 2005;46:634–41.
Forrer F, Krenning EP, Kooij PP, Bernard BF, Konijnenberg M, Bakker WH, et al. Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA0, Tyr3] octreotate. Eur J Nucl Med Mol Imaging. 2009;36:1138–46.
Larson SM, Raubitschek A, Reynolds JC, Neumann RD, Hellstrom K-E, Hellstrom I, et al. Comparison of bone marrow dosimetry and toxic effect of high dose 131I-labeled monoclonal antibodies administered to man. Int J Radiat Appl Instrumen B. 1989;16:153–8.
Siegel J, Wessels B, Watson E, Stabin M, Vriesendorp H, Bradley E, et al. Bone marrow dosimetry and toxicity for radioimmunotherapy. Antibody Immunoconjug Radiopharm. 1990;3:213–34.
Bodei L, Cremonesi M, Grana CM, Chinol M, Baio SM, Severi S, et al. Yttrium-labelled peptides for therapy of NET. Eur J Nucl Med Mol Imaging. 2012;39:93–102.
Kam B, Teunissen J, Krenning E, De Herder W, Khan S, van Vliet E, et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39:103–12.
Sandström M, Garske-Román U, Granberg D, Johansson S, Widström C, Eriksson B, et al. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med. 2013;54:33–41.
Wild D, Fani M, Fischer R, Del Pozzo L, Kaul F, Krebs S, et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J Nucl Med. 2014;55:1248–52.
Emami B, Lyman J, Brown A, Cola L, Goitein M, Munzenrider J, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22.
Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Horsch D, O’Dorisio MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–16.
Garske U, Sandström M, Johansson S, Sundin A, Granberg D, Eriksson B, et al. Minor changes in effective half-life during fractionated 177Lu-octreotate therapy. Acta Oncol. 2012;51:86–96.
Acknowledgments
The authors thank their colleagues from the Department of Nuclear Medicine for their participation in data collection, with particular thanks to the skilled nuclear medicine technicians who performed the imaging studies. U. Haberkorn was supported by a grant from the Klaus-Tschira foundation (grant number 00.198.2012). The manuscript was edited by Inglewood Biomedical Editing.
Compliance with ethical standards
ᅟ
Funding
This study was partially funded by the German Cancer Consortium (DKTK).
Conflicts of interest
None.
Informed consent
For this type of study formal consent is not required.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delker, A., Fendler, W.P., Kratochwil, C. et al. Dosimetry for 177Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging 43, 42–51 (2016). https://doi.org/10.1007/s00259-015-3174-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3174-7