Abstract
Purpose
The PET tracer, 124I-cG250, directed against carbonic anhydrase IX (CAIX) shows promise for presurgical diagnosis of clear-cell renal cell carcinoma (ccRCC) (Divgi et al. in Lancet Oncol 8:304–310, 2007; Divgi et al. in J Clin Oncol 31:187–194, 2013). The radiometal 89Zr, however, may offer advantages as a surrogate PET nuclide over 124I in terms of greater tumor uptake and retention (Rice et al. in Semin Nucl Med 41:265–282, 2011). We have developed a nonlinear immunokinetic model to facilitate a quantitative comparison of absolute uptake and antibody turnover between 124I-cG250 and 89Zr-cG250 using a human ccRCC xenograft tumor model in mice. We believe that this unique model better relates quantitative imaging data to the salient biological features of tumor antibody–antigen binding and turnover.
Methods
We conducted experiments with 89Zr-cG250 and 124I-cG250 using a human ccRCC cell line (SK-RC-38) to characterize the binding affinity and internalization kinetics of the two tracers in vitro. Serial PET imaging was performed in mice bearing subcutaneous ccRCC tumors to simultaneously detect and quantify time-dependent tumor uptake in vivo. Using the known specific activities of the two tracers, the equilibrium rates of antibody internalization and turnover in the tumors were derived from the PET images using nonlinear compartmental modeling.
Results
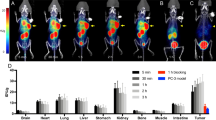
The two tracers demonstrated virtually identical tumor cell binding and internalization but showed markedly different retentions in vitro. Superior PET images were obtained using 89Zr-cG250, owing to the more prolonged trapping of the radiolabel in the tumor and simultaneous washout from normal tissues. Estimates of cG250/CAIX complex turnover were 1.35 – 5.51 × 1012 molecules per hour per gram of tumor (20 % of receptors internalized per hour), and the ratio of 124I/89Zr atoms released per unit time by tumor was 17.5.
Conclusion
Pairwise evaluation of 89Zr-cG250 and 124I-cG250 provided the basis for a nonlinear immunokinetic model which yielded quantitative information about the binding and internalization of radioantibody bound to CAIX on tumor cells in vivo. 89Zr-cG250 is likely to provide high-quality PET images and may be a useful tool to quantify CAIX/cG250 receptor turnover and cG250-accessible antigen density noninvasively in humans.






Similar content being viewed by others
References
Oosterwijk E, Ruiter DJ, Hoedemaeker PJ, Pauwels EK, Jonas U, Zwartendijk J, et al. Monoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer. 1986;38:489–94.
Divgi CR, Pandit-Taskar N, Jungbluth AA, Reuter VE, Gonen M, Ruan S, et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol. 2007;8:304–10.
Divgi CR, Uzzo RG, Gatsonis C, Bartz R, Treutner S, Yu JQ, et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J Clin Oncol. 2013;31:187–94.
Perk LR, Vosjan MJ, Visser GW, Budde M, Jurek P, Kiefer GE, et al. p-Isothiocyanatobenzyl-desferrioxamine: a new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur J Nucl Med Mol Imaging. 2010;37:250–9.
Stillebroer AB, Boerman OC, Desar IM, Boers-Sonderen MJ, van Herpen CM, Langenhuijsen JF, et al. Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur Urol. 2013;64:478–85.
Press OW, DeSantes K, Anderson SK, Geissler F. Inhibition of catabolism of radiolabeled antibodies by tumor cells using lysosomotropic amines and carboxylic ionophores. Cancer Res. 1990;50:1243–50.
Press OW, Shan D, Howell-Clark J, Eary J, Appelbaum FR, Matthews D, et al. Comparative metabolism and retention of iodine-125, yttrium-90, and indium-111 radioimmunoconjugates by cancer cells. Cancer Res. 1996;56:2123–9.
Brouwers AH, Buijs WC, Oosterwijk E, Boerman OC, Mala C, De Mulder PH, et al. Targeting of metastatic renal cell carcinoma with the chimeric monoclonal antibody G250 labeled with (131)I or (111)In: an intrapatient comparison. Clin Cancer Res. 2003;9:3953S–60S.
Rice SL, Roney CA, Daumar P, Lewis JS. The next generation of positron emission tomography radiopharmaceuticals in oncology. Semin Nucl Med. 2011;41:265–82.
Verel I, Visser GW, Boellaard R, Boerman OC, van Eerd J, Snow GB, et al. Quantitative 89Zr immuno-PET for in vivo scouting of 90Y-labeled monoclonal antibodies in xenograft-bearing nude mice. J Nucl Med. 2003;44:1663–70.
Stillebroer AB, Franssen GM, Mulders PF, Oyen WJ, van Dongen GA, Laverman P, et al. ImmunoPET imaging of renal cell carcinoma with (124)I- and (89)Zr-labeled anti-CAIX monoclonal antibody cG250 in mice. Cancer Biother Radiopharm. 2013;28:510–5.
Cheal SM, Punzalan B, Doran M, Evans MJ, Zanzonico P, Lewis JS, et al. Pairwise PET imaging comparisons of 89Zr and 124I labeled cG250 as a basis for non-invasive quantitation of in vivo CAIX receptor binding and internalization in mouse xenografts of clear cell renal carcinoma. Proceedings of the 2012 World Molecular Imaging Congress, Dublin, Ireland, 5–8 Sept 2012. Mol Imaging Biol. 2012;14(2 Suppl):998–2092.
Vosjan MJ, Perk LR, Visser GW, Budde M, Jurek P, Kiefer GE, et al. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat Protoc. 2010;5:739–43.
Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol. 2009;36:729–39.
Anderson CJ, Schwarz SW, Connett JM, Cutler PD, Guo LW, Germain CJ, et al. Preparation, biodistribution and dosimetry of copper-64-labeled anti-colorectal carcinoma monoclonal antibody fragments 1A3-F(ab')2. J Nucl Med. 1995;36:850–8.
Divgi CR, Bander NH, Scott AM, O’Donoghue JA, Sgouros G, Welt S, et al. Phase I/II radioimmunotherapy trial with iodine-131-labeled monoclonal antibody G250 in metastatic renal cell carcinoma. Clin Cancer Res. 1998;4:2729–39.
Kranenborg MH, Boerman OC, de Weijert MC, Oosterwijk-Wakka JC, Corstens FH, Oosterwijk E. The effect of antibody protein dose of anti-renal cell carcinoma monoclonal antibodies in nude mice with renal cell carcinoma xenografts. Cancer. 1997;80:2390–7.
Carlin S, Khan N, Ku T, Longo VA, Larson SM, Smith-Jones PM. Molecular targeting of carbonic anhydrase IX in mice with hypoxic HT29 colorectal tumor xenografts. PloS One. 2010;5:e10857.
Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, Navarro V, Hunter CJ, Bastidas D, et al. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000;60:5237–43.
Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn Jr PA. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72:77–89.
Yao Z, Garmestani K, Wong KJ, Park LS, Dadachova E, Yordanov A, et al. Comparative cellular catabolism and retention of astatine-, bismuth-, and lead-radiolabeled internalizing monoclonal antibody. J Nucl Med. 2001;42:1538–44.
Abou DS, Ku T, Smith-Jones PM. In vivo biodistribution and accumulation of 89Zr in mice. Nucl Med Biol. 2011;38:675–81.
Lam JS, Leppert JT, Belldegrun AS, Figlin RA. Adjuvant therapy of renal cell carcinoma: patient selection and therapeutic options. BJU Int. 2005;96:483–8.
Brouwers A, Verel I, Van Eerd J, Visser G, Steffens M, Oosterwijk E, et al. PET radioimmunoscintigraphy of renal cell cancer using 89Zr-labeled cG250 monoclonal antibody in nude rats. Cancer Biother Radiopharm. 2004;19:155–63.
Geissler F, Anderson SK, Venkatesan P, Press O. Intracellular catabolism of radiolabeled anti-mu antibodies by malignant B-cells. Cancer Res. 1992;52:2907–15.
Brouwers AH, van Eerd JE, Frielink C, Oosterwijk E, Oyen WJ, Corstens FH, et al. Optimization of radioimmunotherapy of renal cell carcinoma: labeling of monoclonal antibody cG250 with 131I, 90Y, 177Lu, or 186Re. J Nucl Med. 2004;45:327–37.
Ritchie M, Tchistiakova L, Scott N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs. 2013;5:13–21.
Mano Y, Suzuki H, Terasaki T, Iwahashi T, Ono K, Naito M, et al. Kinetic analysis of the disposition of MRK16, an anti-P-glycoprotein monoclonal antibody, in tumors: comparison between in vitro and in vivo disposition. J Pharmacol Exp Ther. 1997;283:391–401.
Thurber GM, Weissleder R. Quantitating antibody uptake in vivo: conditional dependence on antigen expression levels. Mol Imaging Biol. 2011;13:623–32.
Rao BM, Lauffenburger DA, Wittrup KD. Integrating cell-level kinetic modeling into the design of engineered protein therapeutics. Nat Biotechnol. 2005;23:191–4.
Xu C, Lo A, Yammanuru A, Tallarico AS, Brady K, Murakami A, et al. Unique biological properties of catalytic domain directed human anti-CAIX antibodies discovered through phage-display technology. PloS One. 2010;5:e9625.
Berguig GY, Convertine AJ, Shi J, Palanca-Wessels MC, Duvall CL, Pun SH, et al. Intracellular delivery and trafficking dynamics of a lymphoma-targeting antibody-polymer conjugate. Mol Pharm. 2012;9:3506–14.
Christiansen JJ, Weimbs T, Bander N, Rajasekaran AK. Differing effects of microtubule depolymerizing and stabilizing chemotherapeutic agents on t-SNARE-mediated apical targeting of prostate-specific membrane antigen. Mol Cancer Ther. 2006;5:2468–73.
O’Donoghue JA, Smith-Jones PM, Humm JL, Ruan S, Pryma DA, Jungbluth AA, et al. 124I-huA33 antibody uptake is driven by A33 antigen concentration in tissues from colorectal cancer patients imaged by immuno-PET. J Nucl Med. 2011;52:1878–85.
Acknowledgments
We thank Ms. Rebekah Cesar and Mr. Ashraf Elzanie for technical assistance, under the City College of New York-Memorial Sloan Kettering Cancer Center Research Training Program in Molecular Imaging, Nanotechnology (ET-CURE). This study was supported in part by The Center for Targeted Radioimmunotherapy and Diagnosis, Ludwig Center for Cancer Immunotherapy, Memorial Sloan Kettering Cancer Center, a training grant from the National Institutes of Health (R25-CA096945), the Geoffrey Beene Cancer Research Center of Memorial Sloan Kettering Cancer Center, and the Center to Reduce Cancer Health Disparity (R21 CA153177-03) (J.O.), and P50 CA 086438-13 (S.M.L.). Technical services provided by the Memorial Sloan Kettering Cancer Center Small-Animal Imaging Core Facility were supported by the National Institutes of Health (R24-CA83084, P30-CA08748, and P50-CA92629).
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 187 kb)
Rights and permissions
About this article
Cite this article
Cheal, S.M., Punzalan, B., Doran, M.G. et al. Pairwise comparison of 89Zr- and 124I-labeled cG250 based on positron emission tomography imaging and nonlinear immunokinetic modeling: in vivo carbonic anhydrase IX receptor binding and internalization in mouse xenografts of clear-cell renal cell carcinoma. Eur J Nucl Med Mol Imaging 41, 985–994 (2014). https://doi.org/10.1007/s00259-013-2679-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2679-1