Abstract
Purpose
PET/MR hybrid scanners have recently been introduced, but not yet validated. The aim of this study was to compare the PET components of a PET/CT hybrid system and of a simultaneous whole-body PET/MR hybrid system with regard to reproducibility of lesion detection and quantitation of tracer uptake.
Methods
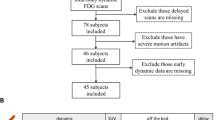
A total of 46 patients underwent a whole-body PET/CT scan 1 h after injection and an average of 88 min later a second scan using a hybrid PET/MR system. The radioactive tracers used were 18F-deoxyglucose (FDG), 18F-ethylcholine (FEC) and 68Ga-DOTATATE (Ga-DOTATATE). The PET images from PET/CT (PETCT) and from PET/MR (PETMR) were analysed for tracer-positive lesions. Regional tracer uptake in these foci was quantified using volumes of interest, and maximal and average standardized uptake values (SUVmax and SUVavg, respectively) were calculated.
Results
Of the 46 patients, 43 were eligible for comparison and statistical analysis. All lesions except one identified by PETCT were identified by PETMR (99.2 %). In 38 patients (88.4 %), the same number of foci were identified by PETCT and by PETMR. In four patients, more lesions were identified by PETMR than by PETCT, in one patient PETCT revealed an additional focus compared to PETMR. The mean SUVmax and SUVavg of all lesions determined by PETMR were by 21 % and 11 % lower, respectively, than the values determined by PETCT (p < 0.05), and a strong correlation between these variables was identified (Spearman rho 0.835; p < 0.01).
Conclusion
PET/MR showed equivalent performance in terms of qualitative lesion detection to PET/CT. The differences demonstrated in quantitation of tracer uptake between PETCT and PETMR were minor, but statistically significant. Nevertheless, a more detailed study of the quantitative accuracy of PETMR and the factors governing it is needed to ultimately assess its accuracy in measuring tissue tracer concentrations.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Cuocolo A, Breatnach E. Multimodality imaging in Europe: a survey by the European Association of Nuclear Medicine (EANM) and the European Society of Radiology (ESR). Eur J Nucl Med Mol Imaging. 2010;37(1):163–7.
Rosenbaum J, Basu S, Beckerman S, Werner T, Torigian DA, Alavi A. Evaluation of diagnostic performance of (18)F-FDG-PET compared to CT in detecting potential causes of fever of unknown origin in an academic centre. Hell J Nucl Med. 2011;14(3):255–9.
Schiepers C, Dahlbom M. Molecular imaging in oncology: the acceptance of PET/CT and the emergence of MR/PET imaging. Eur Radiol. 2011;21(3):548–54.
Blodgett TM, Meltzer CC, Townsend DW. PET/CT: form and function. Radiology. 2007;242(2):360–85.
Freudenberg LS, Rosenbaum SJ, Beyer T, Bockisch A, Antoch G. PET versus PET/CT dual-modality imaging in evaluation of lung cancer. Thorac Surg Clin. 2010;20(1):25–30.
Ell PJ. The contribution of PET/CT to improved patient management. Br J Radiol. 2006;79(937):32–6.
Rosenbaum SJ, Lind T, Antoch G, Bockisch A. False-positive FDG PET uptake – the role of PET/CT. Eur Radiol. 2006;16(5):1054–65.
Bockisch A, Freudenberg LS, Schmidt D, Kuwert T. Hybrid imaging by SPECT/CT and PET/CT: proven outcomes in cancer imaging. Semin Nucl Med. 2009;39(4):276–89.
Chawla SC, Federman N, Zhang D, Nagata K, Nuthakki S, McNitt-Gray M, et al. Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review. Pediatr Radiol. 2010;40(5):681–6.
Mattsson S, Söderberg M. Radiation dose management in CT, SPECT/CT and PET/CT techniques. Radiat Prot Dosimetry. 2011;147(1–2):13–21.
Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14(4):459–65.
Pichler BJ, Judenhofer MS, Catana C, Walton JH, Kneilling M, Nutt RE, et al. Performance test of an LSO-APD detector in a 7-T MRI scanner for simultaneous PET/MRI. J Nucl Med. 2006;47(4):639–47.
Hofmann M, Pichler B, Schölkopf B, Beyer T. Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques. Eur J Nucl Med Mol Imaging. 2009;36 Suppl 1:S93–104.
Judenhofer MS, Catana C, Swann BK, Siegel SB, Jung W-I, Nutt RE, et al. PET/MR images acquired with a compact MR-compatible PET detector in a 7-T magnet. Radiology. 2007;244(3):807–14.
Catana C, Procissi D, Wu Y, Judenhofer MS, Qi J, Pichler BJ, et al. Simultaneous in vivo positron emission tomography and magnetic resonance imaging. Proc Natl Acad Sci U S A. 2008;105(10):3705–10.
Wehrl HF, Judenhofer MS, Wiehr S, Pichler BJ. Pre-clinical PET/MR: technological advances and new perspectives in biomedical research. Eur J Nucl Med Mol Imaging. 2009;36 Suppl 1:S56–68.
Boss A, Bisdas S, Kolb A, Hofmann M, Ernemann U, Claussen CD, et al. Hybrid PET/MRI of intracranial masses: initial experiences and comparison to PET/CT. J Nucl Med. 2010;51(8):1198–205.
Boss A, Stegger L, Bisdas S, Kolb A, Schwenzer N, Pfister M, et al. Feasibility of simultaneous PET/MR imaging in the head and upper neck area. Eur Radiol. 2011;21(7):1439–46.
Schlemmer H-PW, Pichler BJ, Schmand M, Burbar Z, Michel C, Ladebeck R, et al. Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology. 2008;248(3):1028–35.
Delso G, Fürst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52(12):1914–22.
Zaidi H, Ojha N, Morich M, Griesmer J, Hu Z, Maniawski P, et al. Design and performance evaluation of a whole-body Ingenuity TF PET-MRI system. Phys Med Biol. 2011;56(10):3091–106.
Kumar S, Pandey AK, Sharma P, Malhotra A, Kumar R. Optimization of the CT acquisition protocol to reduce patient dose without compromising the diagnostic quality for PET-CT: a phantom study. Nucl Med Commun. 2012;33(2):164–70.
Funama Y, Awai K, Nakayama Y, Kakei K, Nagasue N, Shimamura M, et al. Radiation dose reduction without degradation of low-contrast detectability at abdominal multisection CT with a low-tube voltage technique: phantom study. Radiology. 2005;237(3):905–10.
Martinez-Möller A, Souvatzoglou M, Delso G, Bundschuh RA, Chefd’hotel C, Ziegler SI, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50(4):520–6.
Eiber M, Martinez-Möller A, Souvatzoglou M, et al. Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur J Nucl Med Mol Imaging. 2011;38(9):1691–701.
Hofmann M, Bezrukov I, Mantlik F, Aschoff P, Steinke F, Beyer T, et al. MRI-based attenuation correction for whole-body PET/MRI: quantitative evaluation of segmentation-and atlas-based methods. J Nucl Med. 2011;52(9):1392–9.
Matthies A, Hickeson M, Cuchiara A, Alavi A. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med. 2002;43(7):871–5.
Basu S, Kwee TC, Surti S, Akin EA, Yoo D, Alavi A. Fundamentals of PET and PET/CT imaging. Ann N Y Acad Sci. 2011;1228:1–18.
Zytoon AA, Murakami K, El-Kholy MR, El-Shorbagy E. Dual time point FDG-PET/CT imaging. Potential tool for diagnosis of breast cancer. Clin Radiol. 2008;63(11):1213–27.
Paquet N, Albert A, Foidart J, Hustinx R. Within-patient variability of (18)F-FDG: standardized uptake values in normal tissues. J Nucl Med. 2004;45(5):784–8.
Lee JW, Kim S-K, Lee SM, Moon SH, Kim T-S. Detection of hepatic metastases using dual-time-point FDG PET/CT scans in patients with colorectal cancer. Mol Imaging Biol. 2011;13(3):565–72.
Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Furst S, Martinez-Möller A, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53(6):845–55.
Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: Version 1.0. Eur J Nucl Med Mol Imaging. 2010;37(1):181–200.
Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42(9):1412–7.
Schwenzer NF, Schraml C, Müller M, Brendle C, Sauter A, Spengler W, et al. Pulmonary lesion assessment: comparison of whole-body hybrid MR/PET and PET/CT imaging – pilot study. Radiology. 2012;264(2):551–8.
Mantlik F, Hofmann M, Werner MK, Sauter A, Kupferschläger J, Schölkopf B, et al. The effect of patient positioning aids on PET quantification in PET/MR imaging. Eur J Nucl Med Mol Imaging. 2011;38(5):920–9.
Vriens D, Visser EP, Geus-Oei L-F, Oyen WJG. Methodological considerations in quantification of oncological FDG PET studies. Eur J Nucl Med Mol Imaging. 2009;37(7):1408–25.
Tellmann L, Quick HH, Bockisch A, Herzog H, Beyer T. The effect of MR surface coils on PET quantification in whole-body PET/MR: results from a pseudo-PET/MR phantom study. Med Phys. 2011;38(5):2795–805.
Delso G, Martinez-Möller A, Bundschuh RA, Ladebeck R, Candidus Y, Faul D, et al. Evaluation of the attenuation properties of MR equipment for its use in a whole-body PET/MR scanner. Phys Med Biol. 2010;55(15):4361–74.
MacDonald LR, Kohlmyer S, Liu C, Lewellen TK, Kinahan PE. Effects of MR surface coils on PET quantification. Med Phys. 2011;38(6):2948–56.
Acknowledgments
We would like to thank the whole MR imaging team at IMP Erlangen as well as Ms. Andrea Mühl, our PET/MR technologist, for continuous and unfailing support. The mMR was provided by Siemens Healthcare to the Institute of Medical Physics of the FAU Erlangen-Nuremberg in the framework of a scientific cooperation. The data presented are part of a clinical trial of this device conducted by the Clinic of Nuclear Medicine and the Institute of Radiology.
Conflicts of interest
Michael Beck has no conflicts of interest.
Carl v. Gall is compensated for developing educational presentations on behalf of Siemens Healthcare.
Torsten Kuwert gives lectures on behalf of Siemens Healthcare and has a research cooperation in the field of SPECT/CT. He is the principal investigator of the clinical trial regarding the mMR installed at the IMP Erlangen without any financial compensation.
Michael Lell gives lectures on behalf of Siemens Healthcare and is compensated for developing educational presentations.
Harald H. Quick is head of the MR imaging section at the Institute of Medical Physics Erlangen. The PET/MR system used in this study and installed at the Institute of Medical Physics, Erlangen, was funded through a research cooperation between the University of Erlangen and Siemens Healthcare.
Philipp Ritt has no conflicts of interest.
Daniela Schmidt has no conflicts of interest.
Michael Uder gives lectures on behalf of Siemens Healthcare and is compensated for developing educational presentations for Siemens Healthcare. He has a research cooperation in the field of MRI.
Marco Wiesmüller has no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiesmüller, M., Quick, H.H., Navalpakkam, B. et al. Comparison of lesion detection and quantitation of tracer uptake between PET from a simultaneously acquiring whole-body PET/MR hybrid scanner and PET from PET/CT. Eur J Nucl Med Mol Imaging 40, 12–21 (2013). https://doi.org/10.1007/s00259-012-2249-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2249-y