Abstract
Aim
To evaluate the role of postchemotherapy FDG PET and compare it with other predictive factors in paediatric Hodgkin’s disease (HD).
Materials and methods
In this retrospective study, 98 paediatric patients with HD (enrolled in eight Italian centres) were analysed. Their mean age was 13.8 years (range 5–19 years). A PET scan was performed at the end of chemotherapy and reported as positive or negative on the basis of visual and/or semiquantitative analysis. True outcome was defined as remission or disease on the basis of combined criteria (clinical, instrumental and/or histological) with a mean follow-up period of 25 months. Statistical analyses were performed for the postchemotherapy PET results and other potential predictive factors (age cut-off, stage, presence of bulky masses and therapeutic group) with respect to patient outcome and progression-free survival (PFS).
Results
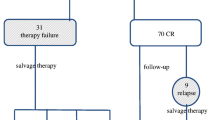
Overall the patients had a mean PFS of 23.5 months (range 4–46 months): 87 achieved remission (88.8%) and 11 showed disease. Of the 98 patients, 17 were positive on postchemotherapy PET . Seven patients (41%) showed disease during follow-up, and relapse occurred in only four out of the 81 patients who were negative on PET (p = 0.0001). Kaplan-Meier analysis demonstrated significant correlations between PFS and the postchemotherapy PET result (p = 0.0001) and a cut-off age at diagnosis of 13.3 years (p = 0.0337), whereas disease stage (p = 0.7404), therapeutic group (p = 0.5240) and presence of bulky masses (p = 0.2208) were not significantly correlated with PFS. Multivariate analysis confirmed a statistically significant correlation with PFS only for the postchemotherapy PET findings (p = 0.0009).
Conclusion
In paediatric HD, age at diagnosis and postchemotherapy PET results are the main predictors of patient outcome and PFS, with FDG PET being the only independent predictive factor for PFS.




Similar content being viewed by others
References
Bar-Sever Z, Keidar Z, Ben-Barak A, et al. The incremental value of 18F-FDG PET/CT in paediatric malignancies. Eur J Nucl Med Mol Imaging. 2007;34:630–7.
Tatsumi M, Miller JH, Wahl RL. 18F-FDG PET/CT in evaluating non-CNS pediatric malignancies. J Nucl Med. 2007;48:1923–31.
Jadvar H, Connolly LP, Fahey FH, Shulkin BL. PET and PET/CT in pediatric oncology. Semin Nucl Med 2007;37:316–331.
Hudson MM, Donaldson SS. Treatment of pediatric Hodgkin’s lymphoma. Semin Hematol. 1999;36:313–23.
Federman N, Feig SA. PET/CT in evaluating pediatric malignancies: a clinician’s perspective. J Nucl Med. 2007;48:1920–2.
Riad R, Omar W, Kotb M, et al. Role of PET/CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:319–29.
Bhatia S, Yasui Y, Robinson LL, et al. High risk of subsequent neoplasm continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol. 2003;23:4386–94.
Furth C, Steffen IG, Amthauer H, et al. Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin's lymphoma: analysis of a prospective multicenter trial. J Clin Oncol. 2009;27:4385–91.
Spaepen K, Stroobants S, Dupont P, et al. Can positron emission tomography with [18F]-fluorodeoxyglucose after first-line treatment distinguish Hodgkin’s disease patients who need additional therapy from others in whom additional therapy would mean avoidable toxicity? Br J Haematol. 2001;115:272–8.
Zinzani PL, Fanti S, Battista G, et al. Predictive role of positron emission tomography (PET) in the outcome of lymphoma patients. Br J Cancer. 2004;91:850–4.
Reinhardt MJ, Herkel C, Altehoefer C, et al. Computer tomography and 18F-FDG positron emission tomography for therapy control of Hodgkin’s and non-Hodgkin’s lymphoma patients: when do we really need FDG-PET? Ann Oncol. 2005;16:1524–9.
Anderson H, Singh N, Miles K. Tumour response evaluation with fluorodeoxyglucose positron emission tomography: research technique or clinical tool? Cancer Imaging. 2010;10:S68–72.
Barnes JA, Lacasce AS, Zukotynski K, et al. End-of-treatment but not interim PET scan predicts outcome in nonbulky limited-stage Hodgkin’s lymphoma. Ann Oncol. 2011;22:910–5
Kobe C, Dietlein M, Franklin J, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first line chemotherapy in advanced-stage Hodgkin lymphoma. Blood. 2008;112(10):3989–94.
Montravers F, McNamara D, Landman-Parker J, et al. [(18)F]FDG in childhood lymphoma: clinical utility and impact on management. Eur J Nucl Med Mol Imaging. 2002;29:1155–65.
Meany H, Gidvani VK, Minniti CP. Utility of PET scans to predict disease relapse in pediatric patients with Hodgkin lymphoma. Pediatr Blood Cancer. 2007;48:399–402.
Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971;31:1860–1.
Burnelli R, Rinieri S, Todesco A, et al. Protocollo AIEOP-LH 2004 per la terapia del linfoma di Hodgkin in età pediatrica: analisi ad interim. XXXV Congresso Nazionale AIEOP, Ancona, 26–28 October 2008. Aricó M, editor. Abstract book, P044. Haematologica 2008;93 Suppl 4:S27.
Stauss J, Franzius C, Pfluger K, et al. Guidelines for 18F-FDG PET and PET-CT imaging in paediatric oncology. Eur J Nucl Med Mol Imaging. 2008;35:1581–8.
Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86.
Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25(5):571–8.
Wahl R, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(5) Suppl 1:122S–50S.
Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(8):561–77.
Kaplan E, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:456–81.
Cox DR. Regression models and life tables (with discussion). J R Stat Soc, Ser B. 1972;34:187–220.
Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70.
Thomson AB, Wallace WHB. Treatment of paediatric Hodgkin’s disease: a balance of risks. Eur J Cancer. 2002;38:468–77.
Smith R, Chen Q, Hudson MM, et al. Prognostic factors for children with Hodgkin’s disease treated with combined-modality therapy. J Clin Oncol. 2003;21:2026–33.
Jerusalem G, Beguin Y, Fassotte MF, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin’s disease and non-Hodgkin’s lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood. 1999;94:429–33.
Weihrauch MR, Re D, Scheidhauer K, et al. Thoracic positron emission tomography using 18F-fluorodeoxyglucose for the evaluation of residual mediastinal Hodgkin disease. Blood. 2001;98:2930–4.
Guay C, Lepine M, Verreault J, Benard F. Prognostic value of PET using 18F-FDG in Hodgkin's disease for posttreatment evaluation. J Nucl Med. 2008;44(8):1225–31.
Depas G, De Barsy C, Jerusalem G, et al. 18F-FDG PET in children with lymphomas. Eur J Nucl Med Mol Imaging. 2005;32:31–8.
Hernandez-Pampaloni M, Takalkar A, Yu JQ, Zhuang H, Alavi A. F-18 FDG-PET imaging and correlation with CT in staging and follow-up of pediatric lymphomas. Pediatr Radiol. 2006;36:524–31.
Schaefer NG, Taverna C, Strobel K, et al. Hodgkin disease: diagnostic value of FDG PET/CT after firstline therapy – is biopsy of FDG-avid lesions still needed? Radiology. 2007;244:257–62.
Edeline V, Bonardel G, Brisse H, et al. Prospective study of 18FFDG PET in pediatric mediastinal lymphoma: a single center experience. Leuk Lymphoma. 2007;48:823–6.
Shankar A, Fiumara F, Pinkerton R. Role of FDG PET in the management of childhood lymphomas – case proven or is the jury still out? Eur J Cancer. 2008;44:663–73.
Levine JM, Weiner M, Kelly KM. Routine use of PET scans after completion of therapy in pediatric Hodgkin disease results in a high false positive rate. J Pediatr Hematol Oncol. 2006;28:711–4.
Eichennauer DA, Bredenfeld H, Haverkamp H, et al. Hodgkin’s lymphoma in adolescents treated with adult protocols: a report from the German Hodgkin study group. J Clin Oncol. 2009;27(36):6079–85.
Dieckmann K, Potter R, Hofmann J, et al. Does bulky disease at diagnosis influence outcome in childhood Hodgkin’s disease and require higher radiation doses? Results from the German-Austrian pediatric multicenter trial DAL-HD-90. Int J Radiat Oncol Biol Phys. 2003;56(3):644–52.
Furth C, Amthauer H, Hautzel H, et al. Evaluation of interim PET response criteria in paediatric Hodgkin's lymphoma – results for dedicated assessment criteria in a blinded dual-centre read. Ann Oncol 2011;22:1198–203.
Meignan M. Interim PET in lymphoma: a step towards standardization. Eur J Nucl Med Mol Imaging. 2010;37:1821–3.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopci, E., Burnelli, R., Guerra, L. et al. Postchemotherapy PET evaluation correlates with patient outcome in paediatric Hodgkin’s disease. Eur J Nucl Med Mol Imaging 38, 1620–1627 (2011). https://doi.org/10.1007/s00259-011-1836-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1836-7