Abstract
Purpose
17-Dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat-shock protein 90 (Hsp90) inhibitor, has been intensively investigated for cancer therapy and is undergoing clinical trials. Human epidermal growth factor receptor 2 (HER-2) is one of the client proteins of Hsp90 and its expression is decreased upon 17-DMAG treatment. In this study, we aimed to noninvasively monitor the HER-2 response to 17-DMAG treatment in xenografted mice.
Methods
The sensitivity of human ovarian cancer SKOV-3 cells to 17-DMAG in vitro was measured by MTT assay. HER-2 expression in SKOV-3 cells was determined by flow cytometry. Nude mice bearing SKOV-3 tumors were treated with 17-DMAG and the therapeutic efficacy was evaluated by tumor size measurement. Both treated and control mice were imaged with microPET using 64Cu-DOTA-trastuzumab and 18F-FDG. Biodistribution studies and immunofluorescence staining were performed to validate the microPET results.
Results
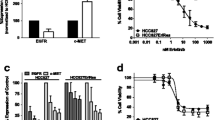
SKOV-3 cells are sensitive to 17-DMAG treatment, in a dose-dependent manner, with an IC50 value of 24.72 nM after 72 h incubation. The tumor growth curve supported the inhibition effect of 17-DMAG on SKOV-3 tumors. Quantitative microPET imaging showed that 64Cu-DOTA-trastuzumab had prominent tumor accumulation in untreated SKOV-3 tumors, which was significantly reduced in 17-DMAG-treated tumors. There was no uptake difference detected by FDG PET. Immunofluorescence staining confirmed the significant reduction in tumor HER-2 level upon 17-DMAG treatment.
Conclusion
The early response to anti-Hsp90 therapy was successfully monitored by quantitative PET using 64Cu-DOTA-trastuzumab. This approach may be valuable in monitoring the therapeutic response in HER-2-positive cancer patients under 17-DMAG treatment.






Similar content being viewed by others
References
Jakob U, Lilie H, Meyer I, Buchner J. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. Implications for heat shock in vivo. J Biol Chem. 1995;270:7288–94. doi:10.1074/jbc.270.13.7288.
Blagosklonny MV, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci U S A. 1996;93:8379–83. doi:10.1073/pnas.93.16.8379.
Clarke PA, Hostein I, Banerji U, Stefano FD, Maloney A, Walton M, et al. Gene expression profiling of human colon cancer cells following inhibition of signal transduction by 17-allylamino-17-demethoxygeldanamycin, an inhibitor of the hsp90 molecular chaperone. Oncogene 2000;19:4125–33. doi:10.1038/sj.onc.1203753.
Burrows F, Zhang H, Kamal A. Hsp90 activation and cell cycle regulation. Cell Cycle 2004;3:1530–6.
Solit DB, Zheng FF, Drobnjak M, Münster PN, Higgins B, Verbel D, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–93.
Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene 2002;21:1159–66. doi:10.1038/sj.onc.1205184.
Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–33.
Schechter AL, Hung MC, Vaidyanathan L, Weinberg RA, Yang-Feng TL, Francke U, et al. The neu gene: an erbB-homologous gene distinct from and unlinked to the gene encoding the EGF receptor. Science 1985;229:976–8. doi:10.1126/science.2992090.
Tan M, Yao J, Yu D. Overexpression of the c-erbB-2 gene enhanced intrinsic metastasis potential in human breast cancer cells without increasing their transformation abilities. Cancer Res. 1997;57:1199–205.
Bacus SS, Ruby SG, Weinberg DS, Chin D, Ortiz R, Bacus JW. HER-2/neu oncogene expression and proliferation in breast cancers. Am J Pathol. 1990;137:103–11.
Wiercioch R, Balcerczak E, Byszewska E, Mirowski M. Uptake of radiolabelled herceptin by experimental mammary adenocarcinoma. Nucl Med Rev Cent East Eur. 2003;6:99–103.
Traish AM, Wotiz HH. Prostatic epidermal growth factor receptors and their regulation by androgens. Endocrinology 1987;121:1461–7.
Fischer U, Kopka L, Brinck U, Korabiowska M, Schauer A, Grabbe E. Prognostic value of contrast-enhanced MR mammography in patients with breast cancer. Eur Radiol. 1997;7:1002–5. doi:10.1007/s003300050240.
Citri A, Alroy I, Lavi S, Rubin C, Xu W, Grammatikakis N, et al. Drug-induced ubiquitylation and degradation of ErbB receptor tyrosine kinases: implications for cancer therapy. EMBO J. 2002;21:2407–17. doi:10.1093/emboj/21.10.2407.
Murakami Y, Mizuno S, Uehara Y. Accelerated degradation of 160 kDa epidermal growth factor (EGF) receptor precursor by the tyrosine kinase inhibitor herbimycin A in the endoplasmic reticulum of A431 human epidermoid carcinoma cells. Biochem J. 1994;301(Pt 1):63–8.
Kelland LR, Sharp SY, Rogers PM, Myers TG, Workman P. DT-Diaphorase expression and tumor cell sensitivity to 17-allylamino, 17-demethoxygeldanamycin, an inhibitor of heat shock protein 90. J Natl Cancer Inst. 1999;91:1940–9. doi:10.1093/jnci/91.22.1940.
Silcox CE, Smith RC, King R, McDannold N, Bromley P, Walsh K, et al. MRI-guided ultrasonic heating allows spatial control of exogenous luciferase in canine prostate. Ultrasound Med Biol. 2005;31:965–70. doi:10.1016/j.ultrasmedbio.2005.03.009.
Sausville EA, Tomaszewski JE, Ivy P. Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr Cancer Drug Targets 2003;3:377–83. doi:10.2174/1568009033481831.
Solit DB, Osman I, Polsky D, Panageas KS, Daud A, Goydos JS, et al. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin Cancer Res. 2008;14:8302–7. doi:10.1158/1078-0432.CCR-08-1002.
Ronnen EA, Kondagunta GV, Ishill N, Sweeney SM, Deluca JK, Schwartz L, et al. A phase II trial of 17-(allylamino)-17-demethoxygeldanamycin in patients with papillary and clear cell renal cell carcinoma. Invest New Drugs 2006;24:543–6. doi:10.1007/s10637-006-9208-z.
Barzilay E, Ben-Califa N, Supino-Rosin L, Kashman Y, Hirschberg K, Elazar Z, et al. Geldanamycin-associated inhibition of intracellular trafficking is attributed to a co-purified activity. J Biol Chem. 2004;279:6847–52. doi:10.1074/jbc.M312799200.
Lang SA, Klein D, Moser C, Gaumann A, Glockzin G, Dahlke MH, et al. Inhibition of heat shock protein 90 impairs epidermal growth factor-mediated signaling in gastric cancer cells and reduces tumor growth and vascularization in vivo. Mol Cancer Ther. 2007;6:1123–32. doi:10.1158/1535-7163.MCT-06-0628.
Smith MA, Morton CL, Phelps DA, Kolb EA, Lock R, Carol H, et al. Stage 1 testing and pharmacodynamic evaluation of the HSP90 inhibitor alvespimycin (17-DMAG, KOS-1022) by the pediatric preclinical testing program. Pediatr Blood Cancer 2008;51:34–41. doi:10.1002/pbc.21508.
Niu G, Chen X. Has molecular and cellular imaging enhanced drug discovery and drug development? Drugs R D 2008;9:351–68. doi:10.2165/0126839-200809060-00002.
McManus DT, Patterson AH, Maxwell P, Humphreys MW, Anderson NH. Fluorescence in situ hybridisation detection of erbB2 amplification in breast cancer fine needle aspirates. Mol Pathol. 1999;52:75–7. doi:10.1136/mp. 52.2.75.
Stomper PC, Budnick RM, Stewart CC. Use of specimen mammography-guided FNA (fine-needle aspirates) for flow cytometric multiple marker analysis and immunophenotyping in breast cancer. Cytometry 2000;42:165–73. doi:10.1002/1097-0320(20000615) 42:3<165::AID-CYTO2>3.0.CO;2-7.
Tokunaga E, Oki E, Nishida K, Koga T, Egashira A, Morita M, et al. Trastuzumab and breast cancer: developments and current status. Int J Clin Oncol. 2006;11:199–208. doi:10.1007/s10147-006-0575-4.
Thrall JH. Personalized medicine. Radiology 2004;231:613–16. 10.1148/radiol.2313040323.
Shepard HM, Lewis GD, Sarup JC, Fendly BM, Maneval D, Mordenti J, et al. Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J Clin Immunol. 1991;11:117–27. doi:10.1007/BF00918679.
Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89:4285–9. doi:10.1073/pnas.89.10.4285.
Bulte JWM, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–99. doi:10.1002/nbm.924.
Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13:95–9. doi:10.1038/nm1467.
Lub-de Hooge MN, Kosterink JG, Perik PJ, Nijnuis H, Tran L, Bart J, et al. Preclinical characterisation of 111In-DTPA-trastuzumab. Br J Pharmacol. 2004;143:99–106. doi:10.1038/sj.bjp.0705915.
Niu G, Cai W, Chen K, Chen X. Non-invasive PET imaging of EGFR degradation induced by a heat shock protein 90 inhibitor. Mol Imaging Biol. 2008;10:99–106. doi:10.1007/s11307-007-0123-2.
Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–9. doi:10.1158/0008-5472.CAN-05-1182.
Lang W, Caldwell GW, Li J, Leo GC, Jones WJ, Masucci JA. Biotransformation of geldanamycin and 17-allylamino-17-demethoxygeldanamycin by human liver microsomes: reductive versus oxidative metabolism and implications. Drug Metab Dispos. 2007;35:21–9. doi:10.1124/dmd.106.009639.
Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X. In vitro and in vivo characterization of 64Cu-labeled Abegrin, a humanized monoclonal antibody against integrin alpha v beta 3. Cancer Res. 2006;66:9673–81. doi:10.1158/0008-5472.CAN-06-1480.
Gossett DR, Bradley MS, Jin X, Lin J. 17-Allyamino-17-demethoxygeldanamycin and 17-NN-dimethyl ethylene diamine-geldanamycin have cytotoxic activity against multiple gynecologic cancer cell types. Gynecol Oncol. 2005;96:381–8. doi:10.1016/j.ygyno.2004.10.009.
Hollingshead M, Alley M, Burger AM, Borgel S, Pacula-Cox C, Fiebig HH, et al. In vivo antitumor efficacy of 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride), a water-soluble geldanamycin derivative. Cancer Chemother Pharmacol. 2005;56:115–25. doi:10.1007/s00280-004-0939-2.
Bast RC Jr, Ravdin P, Hayes DF, Bates S, Fritsche H Jr, Jessup JM, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865–78.
Strahl BD, Allis CD. The language of covalent histone modifications. Nature 2000;403:41–5. doi:10.1038/47412.
Turner BM. Cellular memory and the histone code. Cell 2002;111:285–91. doi:10.1016/S0092-8674(02) 01080-2.
Smith-Jones PM, Solit DB, Akhurst T, Afroze F, Rosen N, Larson SM. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol 2004;22:701–6. doi:10.1038/nbt968.
Smith-Jones PM, Solit D, Afroze F, Rosen N, Larson SM. Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J Nucl Med. 2006;47:793–6.
Orlova A, Tolmachev V, Pehrson R, Lindborg M, Tran T, Sandström M, et al. Synthetic affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res. 2007;67:2178–86. doi:10.1158/0008-5472.CAN-06-2887.
Bernier J. Drug insight: cetuximab in the treatment of recurrent and metastatic squamous cell carcinoma of the head and neck. Nat Clin Pract Oncol. 2008;5:705–13.
Raja SM, Clubb RJ, Bhattacharyya M, Dimri M, Cheng H, Pan W, et al. A combination of trastuzumab and 17-AAG induces enhanced ubiquitinylation and lysosomal pathway-dependent ErbB2 degradation and cytotoxicity in ErbB2-overexpressing breast cancer cells. Cancer Biol Ther. 2008;7:1630–40. doi:10.1158/1535-7163.MCT-07-2409.
Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–7. doi:10.1200/JCO.2007.11.7960.
Acknowledgement
This work was supported by the National Cancer Institute (NCI) P50 CA114747 and a DOD Prostate Postdoctoral Fellowship from Department of Defense (to G. Niu).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niu, G., Li, Z., Cao, Q. et al. Monitoring therapeutic response of human ovarian cancer to 17-DMAG by noninvasive PET imaging with 64Cu-DOTA-trastuzumab. Eur J Nucl Med Mol Imaging 36, 1510–1519 (2009). https://doi.org/10.1007/s00259-009-1158-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1158-1