Abstract
Introduction
In vivo bioluminescence imaging (BLI) is a promising technique for non-invasive tumour imaging. d-luciferin can be administrated intraperitonealy or intravenously. This will influence its availability and, therefore, the bioluminescent signal. The aim of this study is to compare the repeatability of BLI measurement after IV versus IP administration of d-luciferin and assess the correlation between photon emission and histological cell count both in vitro and in vivo.
Materials and methods
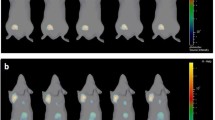
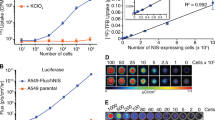
Fluc-positive R1M cells were subcutaneously inoculated in nu/nu mice. Dynamic BLI was performed after IV or IP administration of d-luciferin. Maximal photon emission (PEmax) was calculated. For repeatability assessment, every acquisition was repeated after 4 h and analysed using Bland-Altman method. A second group of animals was serially imaged, alternating IV and IP administration up to 21 days. When mice were killed, PEmax after IV administration was correlated with histological cell number.
Results
The coefficients of repeatability were 80.2% (IV) versus 95.0% (IP). Time-to-peak is shorter, and its variance lower for IV (p < 0.0001). PEmax was 5.6 times higher for IV. A trend was observed towards lower photon emission per cell in larger tumours.
Conclusion
IV administration offers better repeatability and better sensitivity when compared to IP. In larger tumours, multiple factors may contribute to underestimation of tumour burden. It might, therefore, be beneficial to test novel therapeutics on small tumours to enable an accurate evaluation of tumour burden.







Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Kim SA, Kim YC, Kim SW, et al. Antitumor activity of novel indirubin derivatives in rat tumor model. Clin Cancer Res 2007;13(1):253-9.
Rehemtulla A, Stegman LD, Cardozo SJ, et al. Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia 2000;2(6):491-5.
Jenkins DE, Oei Y, Hornig YS, et al. Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin Exp Metastasis 2003;20(8):733-44.
Contag CH, Jenkins D, Contag PR, Negrin RS. Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia 2000;2(1-2):41-52.
Contag PR, Olomu IN, Stevenson DK, Contag CH. Bioluminescent indicators in living mammals. Nat Med 1998;4(2):245-7.
Hastings JW. Chemistries and colors of bioluminescent reactions: a review. Gene 1996;173(1 Spec):5-11.
Wood KV, Lam YA, McElroy WD. Introduction to beetle luciferases and their applications. J Biolumin Chemilumin 1989;4(1):289-301.
Breckpot K, Dullaers M, Bonehill A, et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J Gene Med 2003;5(8):654-7.
Breckpot K, Heirman C, De Greef C, van der Bruggen P, Thielemans K. Identification of new antigenic peptide presented by HLA-Cw7 and encoded by several MAGE genes using dendritic cells transduced with lentiviruses. J Immunol 2004;172(4):2232-7.
De Jonge F, Van Nassauw L, Van Meir F, Miller HR, Van Marck E, Timmermans JP. Temporal distribution of distinct mast cell phenotypes during intestinal schistosomiasis in mice. Parasite Immunol 2002;24(5):225-31.
Frundzhyan V, Ugarova N. Bioluminescent assay of total bacterial contamination of drinking water. Luminescence 2007;22(3):241-4.
Higashi T, Isomoto A, Tyuma I, Kakishita E, Uomoto M, Nagai K. Quantitative and continuous analysis of ATP release from blood platelets with firefly luciferase luminescence. Thromb Haemost 1985;53(1):65-9.
Levin CS. Primer on molecular imaging technology. Eur J Nucl Med Mol Imaging 2005;32(Suppl 2):S325-45.
Contag CH, Spilman SD, Contag PR, et al. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol 1997;66(4):523-31.
Edinger M, Sweeney TJ, Tucker AA, Olomu AB, Negrin RS, Contag CH. Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia 1999;1(4):303-10.
Paroo Z, Bollinger RA, Braasch DA, et al. Validating bioluminescence imaging as a high-throughput, quantitative modality for assessing tumor burden. Mol Imaging 2004;3(2):117-24.
Burgos JS, Rosol M, Moats RA, et al. Time course of bioluminescent signal in orthotopic and heterotopic brain tumors in nude mice. Biotechniques 2003;34(6):1184-8.
Wu JC, Sundaresan G, Iyer M, Gambhir SS. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Molec Ther 2001;4(4):297-306.
Wang W, El-Deiry WS. Bioluminescent molecular imaging of endogenous and exogenous p53-mediated transcription in vitro and in vivo using an HCT116 human colon carcinoma xenograft model. Cancer Biol Ther 2003;2(2):196-202.
Avram MJ, Krejcie TC, Niemann CU, Enders-Klein C, Shanks CA, Henthorn TK. Isoflurane alters the recirculatory pharmacokinetics of physiologic markers. Anesthesiology 2000;92(6):1757-68.
Baba S, Cho SY, Ye Z, Cheng L, Engles JM, Wahl RL. How reproducible is bioluminescent imaging of tumor cell growth? Single time point versus the dynamic measurement approach. Mol Imaging 2007;6(5):315-22.
Edinger M, Cao YA, Hornig YS, et al. Advancing animal models of neoplasia through in vivo bioluminescence imaging. Eur J Cancer 2002;38(16):2128-36.
Sarraf-Yazdi S, Mi J, Dewhirst MW, Clary BM. Use of in vivo bioluminescence imaging to predict hepatic tumor burden in mice. J Surg Res 2004;120(2):249-55.
Scatena CD, Hepner MA, Oei YA, et al. Imaging of bioluminescent LNCaP-luc-M6 tumors: a new animal model for the study of metastatic human prostate cancer. Prostate 2004;59(3):292-303.
Acknowledgement
Marleen Keyaerts is a Ph. D. fellow of the Research Foundation—Flanders (Belgium; FWO). Tony Lahoutte is a Senior Clinical Investigator of the Research Foundation—Flanders (Belgium; FWO). Karine Breckpot is a postdoctoral fellow of the Research Foundation—Flanders (Belgium; FWO). The research at ICMI is funded by the Interuniversity Attraction Poles Programme—Belgian State—Belgian Science Policy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Marleen Keyaerts is a Ph. D. fellow of the Research Foundation—Flanders (Belgium; FWO).
Rights and permissions
About this article
Cite this article
Keyaerts, M., Verschueren, J., Bos, T.J. et al. Dynamic bioluminescence imaging for quantitative tumour burden assessment using IV or IP administration of d-luciferin: effect on intensity, time kinetics and repeatability of photon emission. Eur J Nucl Med Mol Imaging 35, 999–1007 (2008). https://doi.org/10.1007/s00259-007-0664-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0664-2