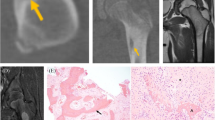
Abstract
The second part of this review, on the benefits of molecular pathology in the diagnosis disease, focuses on the genetics of bone tumors and metabolic disease. Unlike soft tissue tumors, the number of currently exploitable molecular abnormalities for diagnosing bone neoplasms is small, although the same gene rearrangements are found in primitive neuroectodermal tumor/Ewing sarcoma in both skeletal and extraskeletal sites. Compared with soft tissue tumors, genetic abnormalities, which are valuable to diagnosticians in skeletal disease, are often germline and post-zygotic aberrations rather than somatic translocations. In addition, the review highlights the range of disease entities classified as “osteoclast-rich lesions,” some of which harbor germline mutations. It also addresses the importance of phosphate metabolism in skeletal disorders including phosphaturic mesenchymal tumor, vitamin D-resistant rickets, and tumoral calcinosis.






Similar content being viewed by others
References
Wunder JS, Eppert K, Burrow SR, Gokgoz N, Bell RS, Andrulis IL. Co-amplification and overexpression of CDK4, SAS and MDM2 occurs frequently in human parosteal osteosarcomas. Oncogene 1999;18:783–8.
Muller CR, Paulsen EB, Noordhuis P, Pedeutour F, Saeter G, Myklebost O. Potential for treatment of liposarcomas with the MDM2 antagonist Nutlin-3A. Int J Cancer. 2007;121:199–205.
Toledo F, Wahl GM. MDM2 and MDM4: p53 regulators as targets in anticancer therapy. Int J Biochem Cell Biol. 2007;39:1476–82.
Selvarajah S, Yoshimoto M, Ludkovski O, Park PC, Bayani J, Thorner P, et al. Genomic signatures of chromosomal instability and osteosarcoma progression detected by high resolution array CGH and interphase FISH. Cytogenet Genome Res. 2008;122:5–15.
Henderson SR, Guiliano D, Presneau N, McLean S, Frow R, Vujovic S, et al. A molecular map of mesenchymal tumors. Genome Biol. 2005;6:R76.
Man TK, Chintagumpala M, Visvanathan J, Shen J, Perlaky L, Hicks J, et al. Expression profiles of osteosarcoma that can predict response to chemotherapy. Cancer Res. 2005;65:8142–50.
Man TK, Lu XY, Jaeweon K, Perlaky L, Harris CP, Shah S, et al. Genome-wide array comparative genomic hybridization analysis reveals distinct amplifications in osteosarcoma. BMC Cancer 2004;4:45.
Hameetman L, Rozeman LB, Lombaerts M, Oosting J, Taminiau AH, Cleton-Jansen AM, et al. Peripheral chondrosarcoma progression is accompanied by decreased Indian Hedgehog signalling. J Pathol. 2006;209:501–11.
Bovee JV, Cleton-Jansen AM, Taminiau AH, Hogendoorn PC. Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. Lancet Oncol. 2005;6:599–607.
Bovee JV, Cleton-Jansen AM, Wuyts W, Caethoven G, Taminiau AH, Bakker E, et al. EXT-mutation analysis and loss of heterozygosity in sporadic and hereditary osteochondromas and secondary chondrosarcomas. Am J Hum Genet. 1999;65:689–98.
Pannier S, Legeai-Mallet L. Hereditary multiple exostoses and enchondromatosis. Best Pract Res Clin Rheumatol. 2008;22:45–54.
Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumors. Nature. 1989;340:692–6.
Delaney D, Diss TC, Presneau N, Hing S, Berisha F, Idowu BD, et al. GNAS1 mutations occur more commonly than previously thought in intramuscular myxoma. Mod Pathol. 2009;22:718–24.
Okamoto S, Hisaoka M, Ushijima M, Nakahara S, Toyoshima S, Hashimoto H. Activating Gs(alpha) mutation in intramuscular myxomas with and without fibrous dysplasia of bone. Virchows Arch. 2000;437:133–7.
Mazabraud A, Semat P, Roze R. A propos de l'association de fibromyxomes des tissus mous à la dysplasie fibreuse des os. Presse Med. 1967;75:2223–8.
Shenker A, Weinstein LS, Moran A, Pescovitz OH, Charest NJ, Boney CM, et al. Severe endocrine and nonendocrine manifestations of the McCune-Albright syndrome associated with activating mutations of stimulatory G protein GS. J Pediatr. 1993;123:509–18.
Schwindinger WF, Francomano CA, Levine MA. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A. 1992;89:5152–6.
Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–95.
Idowu BD, Al-Adnani M, O'Donnell P, Yu L, Odell E, Diss T, et al. A sensitive mutation-specific screening technique for GNAS1 mutations in cases of fibrous dysplasia: the first report of a codon 227 mutation in bone. Histopathology. 2007;50:691–704.
Fletcher CDM, Krishnan U, Mertens F. Pathology and genetics. Tumors of soft tissue and bone. In: Kleihues P, Sobin LH, editors. World Health Organization classification of tumors. Lyon: IARC Press; 2002.
Kahn LB. Adamantinoma, osteofibrous dysplasia and differentiated adamantinoma. Skeletal Radiol. 2003;32:245–58.
Hazelbag HM, Fleuren GJ, vd Broek LJ, Taminiau AH, Hogendoorn PC. Adamantinoma of the long bones: keratin subclass immunoreactivity pattern with reference to its histogenesis. Am J Surg Pathol. 1993;17:1225–33.
Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994;76:1482–99.
Kamineni S, Briggs TW, Saifuddin A, Sandison A. Osteofibrous dysplasia of the ulna. J Bone Joint Surg Br. 2001;83:1178–80.
Khanna M, Delaney D, Tirabosco R, Saifuddin A. Osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma and adamantinoma: correlation of radiological imaging features with surgical histology and assessment of the use of radiology in contributing to needle biopsy diagnosis. Skeletal Radiol. 2008;37:1077–84.
Lee RS, Weitzel S, Eastwood DM, Monsell F, Pringle J, Cannon SR, et al. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88:658–64.
Pollandt K, Engels C, Kaiser E, Werner M, Delling G. Gsalpha gene mutations in monostotic fibrous dysplasia of bone and fibrous dysplasia-like low-grade central osteosarcoma. Virchows Arch. 2001;439:170–5.
Flanagan AM, Nui B, Tinkler SM, Horton MA, Williams DM, Chambers TJ. The multinucleate cells in giant cell granulomas of the jaw are osteoclasts. Cancer. 1988;62:1139–45.
Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26:265–6.
Oliveira AM, Hsi BL, Weremowicz S, Rosenberg AE, Dal Cin P, Joseph N, et al. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004;64:1920–3.
Oliveira AM, Perez-Atayde AR, Dal Cin P, Gebhardt MC, Chen CJ, Neff JR, et al. Aneurysmal bone cyst variant translocations upregulate USP6 transcription by promoter swapping with the ZNF9, COL1A1, TRAP150, and OMD genes. Oncogene 2005;24:3419–26.
Oliveira AM, Perez-Atayde AR, Inwards CY, Medeiros F, Derr V, Hsi BL, et al. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. Am J Pathol. 2004;165:1773–80.
Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21:153–63.
Cupp JS, Miller MA, Montgomery KD, Nielsen TO, O'Connell JX, Huntsman D, et al. Translocation and expression of CSF1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides. Am J Surg Pathol. 2007;31:970–6.
Ueki Y, Tiziani V, Santanna C, Fukai N, Maulik C, Garfinkle J, et al. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat Genet. 2001;28:125–6.
Jafarov T, Ferimazova N, Reichenberger E. Noonan-like syndrome mutations in PTPN11 in patients diagnosed with cherubism. Clin Genet. 2005;68:190–1.
Beneteau C, Cave H, Moncla A, Dorison N, Munnich A, Verloes A, et al. SOS1 and PTPN11 mutations in five cases of Noonan syndrome with multiple giant cell lesions. Eur J Hum Genet. 2009; doi:10.1038/ejhg.2009.4.
Hanna N, Parfait B, Talaat IM, Vidaud M, Elsedfy HH. SOS1: a new player in the Noonan-like/multiple giant cell lesion syndrome. Clin Genet. 2009;75:568–71.
Van Capelle CI, Hogeman PH, van der Sijs-Bos CJ, Heggelman BG, Idowu B, Slootweg PJ, et al. Neurofibromatosis presenting with a cherubism phenotype. Eur J Pediatr. 2007;166:905–9.
Southgate J, Sarma U, Townend JV, Barron J, Flanagan AM. Study of the cell biology and biochemistry of cherubism. J Clin Pathol. 1998;51:831–7.
Idowu BD, Thomas G, Frow R, Diss TC, Flanagan AM. Mutations in SH3BP2, the cherubism gene, were not detected in central or peripheral giant cell tumors of the jaw. Br J Oral Maxillofac Surg. 2008;46:229–30.
De Lange J, van den Akker HP, van den Berg H. Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:603–15.
Aoki Y, Matsubara Y. Human development and the RAS/MAPK pathway. Seikagaku. 2007;79:34–8.
Croonen EA, van der Burgt I, Kapusta L, Draaisma JM. Electrocardiography in Noonan syndrome PTPN11 gene mutation–phenotype characterization. Am J Med Genet A. 2008;146:350–3.
Shaw AC, Kalidas K, Crosby AH, Jeffery S, Patton MA. The natural history of Noonan syndrome: a long-term follow-up study. Arch Dis Child. 2007;92:128–32.
Tartaglia M, Martinelli S, Stella L, Bocchinfuso G, Flex E, Cordeddu V, et al. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am J Hum Genet. 2006;78:279–90.
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–5.
Fukumoto S, Yamashita T. FGF23 is a hormone-regulating phosphate metabolism—unique biological characteristics of FGF23. Bone 2007;40:1190–5.
Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30.
Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–63.
Consortium A. Autosomal dominant hypophosphatemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–8.
White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–86.
Mikati MA, Melhem RE, Najjar SS. The syndrome of hyperostosis and hyperphosphatemia. J Pediatr. 1981;99:900–4.
Ziran N, Hill S, Wright ME, Kovacs J, Robey PG, Wientroub S, et al. Ribbing disease: radiographic and biochemical characterization, lack of response to pamidronate. Skeletal Radiol. 2002;31:714–9.
Frishberg Y, Topaz O, Bergman R, Behar D, Fisher D, Gordon D, et al. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med. 2005;83:33–8.
Arnold WH. Hereditary bone dysplasia with sarcomatous degeneration. Study of a family. Ann Intern Med. 1973;78:902–6.
Douya H, Yokoyama R, Beppu Y, Hasegawa T. Malignant fibrous histiocytoma associated with diaphyseal medullary stenosis. Clin Orthop Relat Res. 2002;400:211–6.
Hardcastle P, Nade S, Arnold W. Hereditary bone dysplasia with malignant change. Report of three families. J Bone Joint Surg Am. 1986;68:1079–89.
Kenan S, Abdelwahab IF, Hermann G, Klein MJ. Malignant fibrous histiocytoma associated with a bone infarct in a patient with hereditary bone dysplasia. Skeletal Radiol. 1998;27:463–7.
Norton KI, Wagreich JM, Granowetter L, Martignetti JA. Diaphyseal medullary stenosis (sclerosis) with bone malignancy (malignant fibrous histiocytoma): Hardcastle syndrome. Pediatr Radiol. 1996;26:675–7.
Martignetti JA, Desnick RJ, Aliprandis E, Norton KI, Hardcastle P, Nade S, et al. Diaphyseal medullary stenosis with malignant fibrous histiocytoma: a hereditary bone dysplasia/cancer syndrome maps to 9p21–22. Am J Hum Genet. 1999;64:801–7.
Martignetti JA, Gelb BD, Pierce H, Picci P, Desnick RJ. Malignant fibrous histiocytoma: inherited and sporadic forms have loss of heterozygosity at chromosome bands 9p21–22—evidence for a common genetic defect. Genes Chromosomes Cancer 2000;27:191–5.
Ginsberg JP, de Alava E, Ladanyi M, Wexler LH, Kovar H, Paulussen M, et al. EWS-FLI1 and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing's sarcoma. J Clin Oncol. 1999;17:1809–14.
Antonescu CR, Tschernyavsky SJ, Decuseara R, Leung DH, Woodruff JM, Brennan MF, et al. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7:3977–87.
Hisaoka M, Ishida T, Kuo TT, Matsuyama A, Imamura T, Nishida K, et al. Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol. 2008;32:452–60.
Sorensen PH, Lynch JC, Qualman SJ, Tirabosco R, Lim JF, Maurer HM, et al. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2002;20:2672–9.
Acknowledgements
UCL is a partner of the EuroBoNeT consortium, a European Commission granted Network of Excellence for studying the pathology and genetics of bone tumors.
The research was generously funded by Skeletal Cancer Action Trust (SCAT), UK. The work was also supported by the UCLH/UCL Comprehensive Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flanagan, A.M., Delaney, D. & O’Donnell, P. Benefits of molecular pathology in the diagnosis of musculoskeletal disease. Skeletal Radiol 39, 213–224 (2010). https://doi.org/10.1007/s00256-009-0758-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-009-0758-y