Abstract
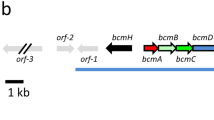
The cefF gene of Nocardia lactamdurans, encoding a functional 2-oxoglutarate-dependent 3′-methylcephem hydroxylase (deacetoxycephalosporin C hydroxylase) has been found to be closely linked to the pcbC gene in the cephamycin C gene cluster. The open-reading frame is 933 bp long and could encode a protein of M r 34366. Introduction of cefF in the cephamycin-non-producer Streptomyces lividans conferred 3′-methylcephem-hydroxylating activity to the transformants but did not result in hydroxylation at carbon 7 of cephamycin. No 3′-methylcephem hydroxylase activity was observed when the cefF gene was introduced in S. lividans in pIJ699 (a vector containing two transcriptional terminators that prevent read-through expression), which suggests that this gene lacks an independent promoter.
Similar content being viewed by others
References
Aharonowitz Y, Cohen G, Martín JF (1992) Penicillin and cephalosporin biosynthetic genes: structure, organization, regulation and evolution. Annu Rev Microbiol 46: 461–496
Baker BJ, Dotzlaf JE, Yeh W-K (1991) Deacetoxycephalosporin C hydroxylase of Streptomyces clavuligerus. J Biol Chem 266:5087–5093
Bankel L, Lindstedt G, Lindstedt S (1972) Thymidine 2′-hydroxylation in Neurospora crassa. J Biol Chem 247:6128–6134
Bibb MJ, Cohen SN (1982) Gene expression in Streptomyces: construction and application of promoter-probe plasmid vectors in Streptomyces lividans. Mol Gen Genet 187:265–277
Bibb MJ, Findlay PR, Johnson MW (1984) The relationship between base composition and the codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene 30:157–166
Coque JJR, Martín JR, Calzada JG, Liras P (1991a) The cephamycin biosynthetic genes pcbAB, encoding a large multidomain peptide synthetase, and pcbC of Nocardia lactamdurans are clustered together in a organization different of the same genes in Acremonium chrysogenum and Penicillium chrysogenum. Mol Microbiol 5:1125–1133
Coque JJR, Liras P, Láiz L, Martín JF (1991b) A gene encoding lysine 6-aminotransferase, which forms the β-lactam precursor α-aminoadipic acid, is located in the cluster of cephamycin biosynthetic genes in Nocardia lactamdurans. J Bacteriol 173:6258–6264
Coque JJR, Martín JF, Liras P (1993a) Characterization and expression in Streptomyces lividans of cefD and cefE genes from Nocardia lactamdurans: the organization of the cephamycin gene cluster differs from that in Streptomyces clavuligerus. Mol Gen Genet 236:453–458
Coque JJR, Malumbres M, Martín JF, Liras P (1993b) Analysis of the codon usage of the cephamycin C producer Nocardia lactamdurans. FEMS Microbiol Lett 110:91–96
Cortés J, Martín JF, Castro JM, Láiz L, Liras P (1987) Purification and characterization of a 2-oxoglutarate linked, ATP-independent deacetoxycephalosporin C synthase of Streptomyces lactamdurans. J Gen Microbiol 133:3165–3174
Demain AL (1983) Biosynthesis of β-lactam antibiotics. In: Demain AL, Solomon NA (eds) Antibiotics containing the β-lactam structure I. Springer, New York, Berlin Heidelberg pp 189–228
Dotzlaf JE, Yeh WK (1986) Copurification and characterization of deacetoxycephalosporin C synthetase/hydroxylase from Cephalosporium acremonium. J Bacteriol 16:1611–1618
Hopwood DA, Bibb MJ, Chater JF, Kieser T, Bruton C, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H (1985) Genetic manipulation of Streptomyces: a laboratory manual. John Innes Institute, Norwich
Jensen SE, Westlake DWS, Wolfe S (1985) Deacetoxycephalosporin C synthase and deacetoxycephalosporin C hydroxylase are two separate enzymes in Streptomyces clavuligerus. J Antibiot (Tokyo) 38:263–265
Katz E, Thompson CJ, Hopwood DA (1983) Cloning and expression of tyrosine gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol 129:2703–2714
Kieser D, Melton RD (1988) Plasmid pIJ699, a multicopy positive selection vector for Streptomyces. Gene 65:83–91
Kovacevic S, Miller JR (1991) Cloning and sequencing of the β-lactam hydroxylase gene (cefF) from Streptomyces clavuligerus: gene duplication may have led to separate hydroxylase and expandase activities in the actinomycetes. J Bacteriol 173:398–400
Kovacevic S, Weigel BJ, Tobin MB, Ingolia TD, Miller JR (1989) Cloning, characterization and expression in Escherichia coli of the Streptomyces clavuligerus gene encoding deacetoxycephalosporin C synthase. J Bacteriol 171:754–760
Kupka J, Shen YQ, Wolfe S, Demain AL (1983) Partial purification and properties of the α-ketoglutarate-linked ring-expansion enzyme of β-lactam biosynthesis by Cephalosporium acremonium. FEMS Microbiol Lett 16:1–6
Majamaa K, Turpeenniemi-Hujanen TM, Latipää P, Günzler V, Hanauske-Abel HM, Hassinen IE, Kivirikko KI (1985) Differences between collagen hydroxylases and 2-oxoglutarate dehydrogenase in their inhibition by structural analogues of 2-oxoglutarate. Biochem J 229:127–133
Martín JF, Liras P (1981) Biosynthetic pathways of secondary metabolites in industrial microorganisms. In: Rehm HJ, Reed G (eds) Biotechnology, vol 1. VCH, Weinheim, pp 211–233
Sambrook, J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory mannual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Samson SM, Dotzlaf JE, Slisz ML, Becker GW, Frank RM van, Veal LE, Yeh WK, Miller JR, Queener SW, Ingolia TD (1987) Cloning and expression of the fungal expandase/hydroxylase gene involved in cephalosporin biosynthesis. Biotechnology 5: 1207–1214
Xiao X, Bowers RJ, Shin H-S, Wolfe S, Demain AL (1991a) Non-radioactive assay and HPLC analysis of cephalosporin C 7-α-methoxylation by cell-free extracts of S. clavuligerus. Appl Microbiol Biotechnol 35:793–797
Xiao X, Wolf S, Demain AL (1991b) Purification and characterization of cephalosporin 7-α-hydroxylase from S. clavuligerus. Biochem J 280:471–474
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Coque, J.J.R., Enguita, F.J., Cardoza, R.E. et al. Characterization of the cefF gene of Nocardia lactamdurans encoding a 3′-methylcephem hydroxylase different from the 7-cephem hydroxylase. Appl Microbiol Biotechnol 44, 605–609 (1996). https://doi.org/10.1007/BF00172492
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00172492