Abstract
The cellulose-rich corncob residue (CCR) is an abundant and renewable agricultural biomass that has been under-exploited. In this study, two strategies were compared for their ability to transform CCR into cello-oligosaccharides (COS). The first strategy employed the use of endo-glucanases. Although selected endo-glucanases from GH9, GH12, GH45, and GH131 could release COS with degrees of polymerization from 2 to 4, the degrading efficiency was low. For the second strategy, first, CCR was efficiently depolymerized to glucose and cellobiose using the cellulase from Trichoderma reesei. Then, using these simple sugars and sucrose as the starting materials, phosphorylases from different microorganisms were combined to generate COS to a level up to 100.3 g/L with different patterns and degrees of polymerization. Using tomato as a model plant, the representative COS obtained from BaSP (a sucrose phosphorylase from Bifidobacterium adolescens), CuCbP (a cellobiose phosphorylase from Cellulomonas uda), and CcCdP (a cellodextrin phosphorylase from Clostridium cellulosi) were shown to be able to promote plant growth. The current study pointed to an approach to make use of CCR for production of the value-added COS.
Key points
• Sequential use of cellulase and phosphorylases effectively generated cello-oligosaccharides from corncob residue.
• Cello-oligosaccharides patterns varied in accordance to cellobiose/cellodextrin phosphorylases.
• Spraying cello-oligosaccharides promoted tomato growth.
Graphical Abstract

Similar content being viewed by others
Introduction
The lignocellulose from agricultural residues is one of the most abundant and renewable biomasses, which has been widely used to produce biofuels, biochemicals, and biomaterials (Ragauskas et al. 2006; Zhang et al. 2007). Among the many agricultural residues is the corncob, which is produced to nearly 88 million tons annually in China (Sun et al. 2022). The corncob has a rich composition (ca. 39%) of xylan (Yang et al. 2009) and is thus commonly used as a feedstock to produce xylitol and xylo-oligosaccharides. During production of these compounds, while xylan in the corncob is extracted by dilute acid treatment, cellulose (another polysaccharide also in rich abundance) and lignin are left as the corncob residue (CCR) (Doiseau et al. 2014; Li et al. 2014). Currently, CCR is not much exploited as a resource but rather used as a feed for animals or simply burned as a waste (Han et al. 2020). However, it is noted that cellulose is further enriched in CCR and accounts for ca. 60%, suggesting that CCR can be used to produce value-added products such as cello-oligosaccharides (COS).
COS are β-1,4-linked glucose oligomers with broad application prospects. COS with a degree of polymerization (DP) from 2 to 6 (G2-G6) are soluble and particularly interesting. In the gut of the humans and animals, COS cannot be degraded by host digestive enzymes but instead utilized by specific gut microbes, thus serving a prebiotic role. Such a prebiotic function has attracted attentions in medicine as well as the animal feed industry (Ávila et al. 2021; Cangiano et al. 2020; Jiao et al. 2014; Uyeno et al. 2015; Zhong et al. 2020). In this regard, administration of COS is observed to be accompanied with an inhibition of growth of pathogenic microorganisms such as the Clostridium spp. and lowers the total cholesterol and neutral fat concentration in the liver in cattle (Ávila et al. 2021; Uyeno et al. 2015). In addition, due to its low calorie and physicochemical properties such as resistance to heat and acidic pH, COS can be incorporated into food as a bulking agent. Furthermore, COS have displayed considerable potentials in other industries such as the cosmetics and crop industries (Ávila et al. 2021).
As cellulose is a long chain of β-1,4-linked glucose polymer, one strategy to produce COS is naturally to make use of degradation of lignocellulosic biomass rich in cellulose, which is usually achieved by applying the microbial cellulase. In nature, many microbes that can utilize cellulose as a carbon source develops a suite of cellulase that can depolymerize cellulose into cello-oligosaccharides and then to glucose. The most widely utilized industrial cellulase is produced by Trichoderma reesei, which is composed of cellobiohydrolases (CBHs, including CBH1 and CBH2), endo-glucanases (EGs), and β-glucosidases (BGLs), which act in concert to hydrolyze cellulose. The CBHs predominantly releases cellobiose, and BGLs release glucose as the end products (Karnaouri et al. 2019). Many EGs, and particularly processive EGs belonging to the GH5, GH6, GH7, and GH9 families, also release cellobiose as the main end products (Amore et al. 2013). Some EGs within GH44 and GH45 families can, however, release COS with larger degree of polymerization (Liu et al. 2010; Warner et al. 2011).
Another route to COS production is the bottom-up synthesis fulfilled by combinatorial action of phosphorylases. This strategy involves the coupled reactions catalyzed by sucrose phosphorylase (SP; EC 2.4.1.7) (De Winter et al. 2011), cellobiose phosphorylase (CbP; EC 2.4.1.20) (Schwaiger et al. 2022), and cellodextrin phosphorylase (CdP; EC 2.4.1.49) (Zhong and Nidetzky 2020). SP releases from sucrose the α-D-glucose 1-phosphate (αG1-P), which serves as a high-energy intermediate that can be linked to glucose and short-chain COS to form cellobiose and COS with higher degrees of polymerization. One advantage of this way of biosynthesis is the ability to control the degree of polymerization by adjusting the amounts and ratio of the enzymes used in the reactions. Currently, this strategy has been exploited for synthesis of cellobiose and COS from glucose (Schwaiger et al. 2020; Zhong et al. 2019). However, there is no report regarding using this strategy for produce COS from agricultural residues.
In this study, the endo-glucanase-catalyzed depolymerization and cellulase/phosphorylase-based degradation-synthesis reactions were compared for their ability to produce COS from the agricultural residue CCR. Unlike other feedstocks, CCR appeared to be very recalcitrant to treatment by selected EGs. In contrast, a combination of T. reesei cellulase hydrolysis and bottom-up synthesis was discovered to produce COS efficiently. Using tomato as a model plant, the function of CCR-derived COS produced by selected enzymes was affirmed by observing their ability to stimulate plant growth.
Materials and methods
Microbial strains and culture conditions
The Escherichia coli Trans1-T1 used for plasmid construction, maintenance, and propagation was purchased from TransGen (Beijing, China). The endo-glucanases and phosphorylases were recombinantly expressed either in the E. coli BL21(DE3) (Invitrogen, Carlsbad, CA) or the Pichia pastoris GS115 (Thermo Fisher Science, Lafayette, LA, USA). The T. reesei strain SUS4, which is a mutant of QM9414 (ATCC 26921) expressing the cellobiohydrolases, endo-glucanases, and β-glucosidase, was maintained in our lab (Gao et al. 2018).
The Luria–Bertani (LB) medium, which contained yeast extract (0.5%), peptone (1%), and sodium chloride (1%), was used for cultivation of E. coli. Pichia pastoris was cultured in the yeast extract peptone dextrose medium (YPD, containing 1% yeast extract, 2% peptone, and 2% glucose) at 30 °C for cell propagation. Trichoderma reesei was maintained on potato dextrose agar (PDA) at 28 °C for sporulation. For cellulase production, the T. reesei strains were grown in a minimal medium (MM) containing (NH4)2SO4, 5.0 g/L; KH2PO4, 15 g/L; MgSO4, 0.6 g/L; CaCl2, 0.6 g/L; FeSO4·7H2O, 0.005 g/L; MnSO4·H2O, 0.0016 g/L; ZnSO4·7H2O, 0.0014 g/L; CoCl2, 0.002 g/L. MM was supplemented with glucose (2%) for mycelial growth or Avicel crystalline cellulose (2%) for secretory expression of cellulase.
Gene cloning and plasmid construction
Six microbial cellulases classified into GH9, GH12, GH45, and GH131 families were selected for recombinant expression and tested for their ability to hydrolyze CCR. These include CpCel9 (Cphy_3367, GH9, GenBank accession number: NC_010001.1) from Clostridium phytofermentans (Warner et al. 2011), CcCel9M (GH9, GenBank accession number: AF316823) from Clostridium cellulolyticum (Belaich et al. 2002), NcCel45A (GH45, GenBank accession number: XM_952014.1) from Neurospora crassa OR74A (Kadowaki et al. 2015), NfCel12A (GH12, GenBank accession number: KX507179) from Neosarorya fischeri P1 (Yang et al. 2017), MtCel45 (GH45, GenBank accession number: XP_003659323) from Myceliophera thermophila (Berto et al. 2019), and ChCel131A (GH131, GenBank accession number: CCF46974.1) from Colletotrichum higginsianum (Anasontzis et al. 2019). CpCel9 and CcCel9M were synthesized by Genewiz (Suzhou, Jiangsu Province, China) and then cloned into the plasmid pET-28a( +) (Invitrogen, Carlsbad, CA, USA) between EcoRI and NotI. The NfCel12A gene was amplified from the genomic DNA of N. fischeri P1 and cloned into the EcoRI and NotI sites of plasmid pPIC9 (Invitrogen, Carlsbad, CA). The genes encoding MtCel45 (codon optimized for P. pastoris, GenBank accession number: OQ801395), NcCel45A, and ChCel131A (codon optimized for P. pastoris, GenBank accession number: OQ801396) were also synthesized by Genewiz but cloned into the plasmid pPICZα-A (Invitrogen, Carlsbad, CA) between the EcoRI and NotI restriction sites.
Five phosphorylases were selected in this study to test their ability to transform glucose, cellobiose, and CCR-derived sugars into COS. These included the sucrose phosphorylase from Bifidobacterium adolescens (BaSP, GenBank accession number AF543301.1) (Mirza et al. 2006), cellobiose phosphorylase (CuCbP, AAQ20920.1) (De Groeve et al. 2009), and its mutant OCP2 (Ubiparip et al. 2020) from Cellulomonas uda, cellodextrin phosphorylase from Clostridium cellulosi (CcCdP, CDZ24361.1) (Zhong et al. 2019), and Thermosipho africanus (TaCdP, ACJ76363.1) (Wu et al. 2017). The genes encoding these enzymes were codon-optimized according to the codon bias of E. coli and synthesized by Genewiz. The GenBank accession numbers of the codon-optimized nucleotide sequences for these five phosphorylases were BaSP (OQ801390), CuCbP (OQ801391), OCP2 (OQ801392), CcCdP (OQ801393), and TaCdP (OQ801394), respectively. All genes were cloned into the plasmid pET-28a( +) between EcoRI and NotI, yielding the corresponding phosphorylase-expression plasmids (pET-BaSP, pET-CuCbP, pET-OCP2, pET-CcCdP, and pET-TaCdP, respectively).
Recombinant enzyme expression
The pET28a-based recombinant plasmids with correct inserts were individually transformed into E. coli BL21 (DE3) by chemical transformation. A single colony of the BL21(DE3) strain transformed with one of the endo-glucanases (CpCel9/CcCel9M) and phosphorylases (BaSP, CuCbP, OCP2, CcCdP, and TaCdP) was inoculated into 5 mL of LB medium supplemented with 50 mg/ml of kanamycin as the seed cell culture, which was shaken at 220 rpm at 37 °C overnight. This seed culture was then transferred into 300 mL of the same medium until the optical density at 600 nm (OD600) reached 0.6. Protein expression was induced by adding isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 0.1 mM, and the culture was continued at 16 °C for 16 h. The cells were harvested by centrifugation at 6000 rpm for 10 min at 4 °C.
For P. pastoris-based expression, the recombinant pPIC9 plasmid encoding NfCel12A was linearized, electroporated into GS115 competent cells, and spread onto minimal dextrose medium (MD) plates for selection of positive colonies. Instead, recombinant pPICZα-A plasmids encoding one of three proteins (MtCel45, NcCel45A, and ChCel131A) were linearized by SacI, electroporated into GS115 competent cells, and spread onto zeocin containing yeast peptone glucose (YPD) agar substrate. For expressing these four endo-glucanases, the P. pastoris strains integrated with the transformed genes were cultivated in 200 mL of buffered glycerol-complex medium (BMGY, containing yeast extract 10 g/L, peptone 20 g/L, glycerol 10 mL, biotin 4 × 10−4 g/L, and yeast nitrogen base (YNB) 13.4 g/L) containing 100 μg/mL zeocin overnight at 30 °C and shaken at 200 rpm for 48 h. Then, the culture medium was changed to buffered methanol-complex medium (BMMY, containing yeast extract 10 g/L, peptone 20 g/L, biotin 4 × 10−4 g/L, YNB 13.4 g/L, and methanol 0.5%) according to the Pichia Fermentation Process Guide (Thermo Fisher Scientific, Lafayette, CO) and the cell density was adjusted to an OD600 of 1.0. The culture was continued at 30 °C for 72 h with shaking at 200 rpm. Methanol was added to the culture every 24 h to maintain a final concentration of 0.5% (v/v) for induction of expression of the recombinant enzymes. At the end of culture, the fermentation broth was centrifuged at 4000 rpm for 5 min and the clear culture supernatant was collected for further purification.
Protein purification
The cell pellets of E. coli expressing recombinant enzymes were washed with 100 mM 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid (HEPES) buffer (pH7.0) and then re-suspended in the same buffer for sonication. The cell lysate was centrifuged at 12,000 rpm for 10 min to separate the supernatant (including the crude enzymes) and the cell debris. For purification of the endo-glucanases and phosphorylases, the supernatant was applied to a nickel-charged resin column (Takara, Shiga, Japan). The HEPES buffers with gradient imidazole concentrations (5, 20, 50, 100, 200, and 300 mM) were used to elute the bound proteins. The elution fractions containing primarily the target proteins, as revealed by SDS-PAGE, were pooled and considered as the purified enzymes. The purified proteins were concentrated using a centrifugal filtration tube with a 10 kDa molecular weight cut-off and the buffer was changed through ultrafiltration centrifugation. Fractions containing the respective enzymes were collected and stored at 4 °C for further application.
For enzymes expressed in P. pastoris, the induced yeast in shake flask fermentation was centrifuged at 6000 rpm for 10 min and the supernatants containing the recombinant enzymes were collected. Then, the supernatant was concentrated and buffer-changed to 50 mM HAc-NaAc buffer (pH 6.0) using a 5-kDa centrifugal filter. The enzymes were further purified by the immobilized affinity chromatography as described above.
Hydrolysis of cellulose and CCR by recombinant endo-glucanases
Sodium carboxymethyl cellulose (CMC) representing soluble cellulose and Avicel representing crystalline cellulose were used as model cellulosic substrates. These two substrates, together with CCR, were individually incubated with one of the purified recombinant endo-glucanases CpCel9, CcCel9M, NfCel12A, MtCel45, NcCel45A, and ChCel131A in a 50 mM of HAc-NaAc buffer solution (pH 6.0) at 50 °C for 24 h. In the reaction mixture, 0.5 μM each of the endo-glucanases was incubated with 1% substrate and the reaction was shaken at 900 rpm. After the reaction, the samples were centrifuged to separate the supernatants, which were further filtered through a 3-kDa filter tube. The production of COS produced was determined using the high-performance anion-exchange chromatographic system coupled with a pulsed amperometric detection (HPAEC-PAD) according to a method previously described (Westereng et al. 2016). HPAEC-PAD was analyzed in a Thermo Chromeleon high performance liquid chromatographic analysis system equipped with a CarboPacTM PA100 (4.0 × 250 mm, 8.5 μm) column (Thermo Fisher, Waltham, MA, USA). The mobile phase A was water, and mobile phase B was 1 mol/L NaOH. The chromatographic analysis program followed 100% A elution for 4 min, 0–100% B elution for 16 min, and 100% B elution for 6 min.
Phosphorylase-catalyzed COS synthesis from glucose and cellobiose
Purified enzymes were used in all reactions. In order to determine the ability of cellobiose phosphorylase and cellodextrin phosphorylase to synthesize COS directly from glucose, 100 mM sucrose and 50 mM glucose were used as substrates of BaSP and CuCbP (each at 0.2 mg/mL). In addition, 100 mM sucrose and 50 mM cellobiose were also tested as the substrates for COS synthesis using CDP. All reactions were carried out at 45 °C. A HEPES buffer (100 mM, pH 7.0) was used. In the reaction including BaSP (0.2 mg/mL), CuCbP (0.4 mg/mL), and one of the CdPs (0.4 mg/mL), the substrate concentrations were set as 100 mM phosphate, 500 mM sucrose, and 200 mM glucose. The reaction was terminated after 2 h, and the samples were heated (95 °C, 5 min) to inactivate the enzymes, and analyzed by HPAEC-PAD. Unless otherwise mentioned, the reactions were performed in a total volume of 1.0 mL and scaled up to 20 mL in certain cases.
Preparation of glucose/cellobiose mixture by hydrolyzing CCR with the T. reesei cellulase
The CCR (a kindly gift from Yuanlong Biotech., Henan Province, China) composed of ~ 60% cellulose and ~ 40% lignin was used for COS production in this study. Preparation of glucose/cellobiose mixture was carried out by hydrolyzing CCR using the T. reesei cellulase. Briefly, the T. reesei cellulase at a final concentration of 1 mg/mL was added to 80 g/L of CCR re-suspended with 100 mM of the HEPES buffer (pH7.0) in a total reaction volume of 50 mL. The samples were incubated at pH5.0 by shaking at 180 rpm at 50 °C for 120 h. Samples were taken periodically for analysis of the concentrations of glucose and other sugars in the supernatant. The cellulose conversion rate (as estimated by the generation of glucose) was calculated by the following equation: Conversion (%) = [(Cg × V × 0.9)/(M × 60%)] × 100, where Cg was the concentrations of glucose equivalents (g/L), V was the total volume of reaction system (L), and M was the total amount of substrate (g).
Synthesis of COS from the CCR hydrolysate
Synthesis of COS from the CCR hydrolysate was carried out by incubating 0.2 mg/mL SP and 0.4 mg/mL each of CbP and CdPs with the CCR hydrolysate (containing 155.4 mM of glucose and 8.8 mM of cellobiose) in 100 mM HEPES buffer (pH 7.0) supplemented with 50 mM phosphate and 400 mM of sucrose. The mixture was incubated at 45 °C for 2 h, and then, the reaction was terminated by boiling for 10 min. The reaction products were centrifuged to remove any denatured enzymes. Then, the supernatant was used for HPAEC-PAD analysis of generated cello-oligosaccharides.
Effect of COS spraying on growth of tomato
The tomato seed used in this study was “FenGuan #2” (from Shandong Shouhe Seed Industry Co., Ltd), and COS was prepared by phosphorylase-catalyzed reaction using the CCR hydrolysate. The tomato seeds were firstly treated by soaking with different concentrations of COS (0, 40, 80, and 160 mg/L). Briefly, the seeds were soaked in cold water for 20–30 min, during which the bad dried seeds were removed. The cold water was replaced with warm water (55 °C), and the seeds were gently stirred for 30 min and then washed with clean water. The tomato seeds were further soaked for 6 h in the COS solutions at different concentrations. Then, the COS was removed by washing with water. The treatments were repeated for 4 times.
Selected seeds with full granules were sown on 15 × 13 cm matrix basin with 5 capsules per basin. The seeds were placed in the dark room for germination, and the ambient temperature was set at 28 °C. On day 10 after sowing of the tomato seeds, one representative tomato seedling was kept in each pot for subsequent experiments. When the seedlings grew to two leaves, COS solutions at different concentrations (0, 40, 80, and 160 mg/L) were used to spray the whole plant leaf surface once a day. Each treatment has 4 replicates. Four consecutive sprays were conducted with an even interval of 2 d.
Results
Selected endo-glucanases released minor amounts of COS from CCR
Many endo-glucanases release cellobiose and glucose, but not COS with higher degrees of polymerization, as the end products from cellulose (Haq et al. 2015; Su et al. 2012; Yi et al. 2013). Six endo-glucanases from the GH families of 5, 9, 12, and 45 were reported to generate COS with higher degrees of polymerization and selected as candidate enzymes in this study. Thus, these enzymes included CpCel9 from C. phytoplasmans (Warner et al. 2011), CcCel9M from C. cellulolyticum (Belaich et al. 2002), NfCel12A from N. fischeri P1 (Yang et al. 2017), NcCel45A from N. crassa OR74A (Kadowaki et al. 2015), MtCel45 from M. thermophila (Berto et al. 2019), and ChCel131A from C. higginsianum (Anasontzis et al. 2019). The endo-glucanases were recombinantly produced either in E. coli (CpCel9 and CcCel9M) or in P. pastoris (NfCel12A, NcCel45A, MtCel45, and ChCel131A) and purified to near homogeneity (Fig. 1A). When CMC was used, all enzymes were active on this substrate and produced glucose to cellotetraose as the end products in a 24-h incubation. The productivity of CpCel9 was the highest, generating COS with a total amount of 66.18 mg/g CMC and cellobiose as the major end product. CcCel9M, NfCel12A, and MtCel45 produced similar levels of COS ranging from 25 to 30 mg/g CMC. NcCel45A and ChCel131A produced lower levels of COS (Fig. 1B). The COS generated by CpCel9, CcCel9M, and NfCel12A were much less when the crystalline Avicel cellulose was used as the substrate (Fig. 1C). This is reasonable since the recalcitrance increases in crystalline cellulose compared with CMC. However, MtCel45, NcCel5A, and ChCel131A released more COS from Avicel than from CMC, suggesting that these endo-glucanases could favor crystalline cellulose as the substrate (Fig. 1C). When CCR was used as the substrate, although CpCel9 produced the highest amount of COS, unlike CMC, the major product turned to cellobiose and the yield of COS was still low. Moreover, all other endo-glucanases were inefficient in releasing COS (Fig. 1D). The further lowered efficiency in hydrolysis of CCR by these solely acting endo-glucanases was most likely from the recalcitrance of crystalline cellulose and the hindrance by the residual lignin. As simultaneous use of two enzymes could synergize in degrading recalcitrant cellulose, the two family 9 endo-glucanases CpCel9 and CcCel9M that released comparatively higher amounts of COS in CCR degradation and also expressed well were individually combined with one of the rest enzymes (but not NfCel12A with poor performance in CCR degradation) at an equal molar ratio. However, no synergy was observed for any of the combinations in the HPAEC-PAD analysis (Supplemental Fig. S1). In addition, mixing Fe2+ and H2O2 led to a Fenton reaction generating free radicals that can destruct the lignin barrier. However, introducing the Fenton reaction did not result into significantly improved release of reducing sugars (data not shown).
Characterization of the endo-glucanases for their ability to release COS. A SDS-PAGE analysis of the six purified endo-glucanases. Lane 1: molecular mass marker; 2: CpCel9; 3: CcCel9M; 4: NfCel12A; 5: NcCel45A; 6: MtCel45; 7: ChCel131A. The COS released from CMC (B), Avicel (C), and CCR (D) by each of the six endo-glucanases. G: glucose; G2: cellobiose; G3: cellotriose; G4: cellotetraose; G5: cellopentaose; and G6: cellohexaose. The reactions were carried out for 24 h under the optimal conditions for each of the enzymes
Combining sucrose phosphorylase and cellobiose/cellodextrin phosphorylases enabled synthesis of COS from glucose and cellobiose
In addition to the endo-glucanase-catalyzed degradation, another strategy, i.e., the bottom-up strategy, to synthesize COS from simple sugars including the glucose and cellobiose, was also examined in this study as an alternative route towards COS production from CCR. This is because that although the selected endo-glucanase did not catalyze satisfactory CCR degradation for COS production, there are many known cellulase mixtures that commonly release glucose and cellobiose as the end products from cellulose degradation (Herr 1980; Sheng et al. 2016), and these two simple sugars could be utilized by phosphorylases to synthesize COS. For this purpose, one sucrose phosphorylases (BaSP from B. adolescens) and four cellobiose/cellodextrin phosphorylases (CuCbP from C. uda, OCP2 (a mutant of CuCbP), CcCdP from C. cellulosi, and TaCdP from T. africanus) were selected for heterologous expression in E. coli. The recombinant enzymes were produced by inducing the E. coli with IPTG and further purified by immobilized metal affinity chromatograph. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) indicated that these proteins were purified to near homogeneity and they were used for further analysis (Fig. 2A).
Production of cellobiose and other COS by the purified phosphorylases from pure sugars. A SDS-PAGE analysis of the five purified phosphorylases. Lane 1: molecular mass marker; 2: BaSP; 3: CuCbP; 4: OCP2; 5: CcCdP; 6: TaCdP. B Phosphorylase-catalyzed synthesis of COS from glucose. CuCbP and OCP2 were individually incubated with glucose in presence of BaSP and sucrose. C Phosphorylase-catalyzed synthesis of COS from cellobiose. OCP2, CcCdP, and TaCdP were individually incubated with cellobiose in presence of BaSP and sucrose. D Phosphorylase-catalyzed synthesis of COS from a mixture of glucose and cellobiose. BaSP and CuCbP were incubated with sucrose and one of the three phosphorylases (OCP2, CcCdP, and TaCdP). G: glucose; G-1-P: glucose-1-phosphate; F: fructose; S: sucrose; G2: cellobiose; G3: cellotriose; G4: cellotetraose; G5: cellopentaose; G6: cellohexaose. The numbers on the peaks were the concentrations (in g/L) of the corresponding sugars
The ability of the recombinant enzymes in synthesizing COS from glucose and cellobiose was evaluated. First, in the presence of 50 mM of glucose and 100 mM of sucrose (serving as the provider of αG1-P), CuCbP produced 41.2 mM of cellobiose and OCP2 produced 19.9 mM of cellobiose and 22.2 mM of cellotriose, respectively, after 4 h of incubation (Fig. 2B). With cellobiose (50 mM) and sucrose (100 mM) as the starting sugars, OCP2 could produce 43.0 mM of cellotriose and 1.5 mM of cellotetraose. Under the same condition, CcCdP produced 7.3 mM of cellotriose, 8.1 mM of cellotetraose, 11.5 mM of cellopentaose, and 7.9 mM of cellohexaose, respectively. Unexpectedly, TaCdP produced three major peaks with retention time not matching any of the COS standards, suggesting the possibility of forming isomerized products during the reaction (Fig. 2C).
Then, BaSP and CuCbP were combined with one of the three enzymes that catalyze synthesis of COS with higher degrees of polymerization (OCP2, CcCdP, and TaCdP) to produce COS from glucose. When 200 mM of glucose and 500 mM of sucrose were used, OCP2 generated 50.0 mM of cellobiose, 132.4 mM of cellotriose, and 6.5 mM of cellotetraose, respectively. In contrast, CcCdP generated a series of COS with degrees of polymerization ranging from 2 to 6. Specifically, there were 26.9 mM of cellobiose, 42.6 mM of cellotriose, 37.1 mM of cellotetraose, 40.8 mM of cellopentaose, and 27.9 mM of cellohexaose, respectively. Similarly, the three major peaks in the TaCdP-catalyzed reaction did not match any of the COS standards (Fig. 2D).
Synthesis of COS from the CCR hydrolysate
The T. reesei cellulase was produced as a mixture of enzymes, containing mainly synergistically acting cellobiohydrolases and endo-glucanases. As expected, incubation of the T. reesei cellulase generated mainly glucose (91%) and cellobiose (9%) from CCR after 120 h of reaction (Supplemental Fig. S2). COS with higher degrees of polymerization were not discovered in the reaction products. The higher efficiency could be ascribed to co-operation of the myriad of T. reesei enzymes.
As we have demonstrated that combining sucrose phosphatase with one or more cellobiose/cellodextrin phosphatase could lead to generation of COS from glucose and cellobiose mixture, we then started to test if COS could also be produced for the CCR hydrolysate. CcCdP was chosen as a representative cellodextrin phosphorylase for subsequent synthesis of soluble COS from CCR hydrolysates as its product contained a series of COS (G2–G6) with similar concentrations to each other. CcCdP has been used successfully before to produce G2–G6 (Zhong et al. 2019; Zhong and Nidetzky 2020). Moreover, BaSP, CuCbP, and CcCdP have similar pH and temperature preferences, which is an additional advantage for product formation (Cerdobbel et al. 2010; Zhong et al. 2019).
The concentration of sucrose was maintained at a level so that the molar ratio of glucose and sucrose in the system was kept at 1:2.5. The pH of the hydrolysate was adjusted to that (pH 7.0) favoring the activity of selected phosphorylases (Zhong et al. 2019). In the cascade phosphorylase reactions using the CCR hydrolysate as the starting material, a large quantity of COS was obtained and the ratios of sugar transformation for all the three groups of reactions are up to above 90%. Importantly, the oligosaccharides composition profiles were similar to those obtained using the pure glucose and sucrose as the substrate. These two facts clearly indicated that the residual lignin compounds in the CCR did not have an obviously negative impact on producing COS. Indeed, the yields of total soluble COS were 69.0 g/L (for OCP2, representing a total of 35.6, 106.1, and 5.0 mM of G2-G4) and 100.3 g/L (for CcCdP, representing a total of 13.1, 16.9, 27.6, 38.5, and 37.3 mM of G2–G6), respectively (Fig. 3).
Production of COS by phosphorylases from CCR hydrolysate. The corncob residue (CCR) was hydrolyzed by the T. reesei cellulase mainly to glucose and cellobiose, which, in the presence of BaSP and sucrose, were transformed to COS by incubating CuCbP with one of the three phosphorylases (OCP2, CcCdP, and TaCdP). G: glucose; G-1-P: glucose-1-phosphate; F: fructose; S: sucrose; G2: cellobiose; G3: cellotriose; G4: cellotetraose; G5: cellopentaose; G6: cellohexaose. The numbers on the peaks were the concentrations (in g/L) of the corresponding sugars
Effect of COS on tomato growth
In agriculture practice, oligosaccharides can be used as phytoalexins to improve the resistance of crops to biological stress (Choudhary et al. 2017; Sathiyabama and Manikandan 2016; Zhao et al. 2007). Some researchers also found that some plants applying oligosaccharides displayed larger biomass accumulation. At present, the exact mechanism of oligosaccharides affecting plant growth and development is still unclear. However, it has been proposed that chito-oligosaccharides could activate the transcription of genes mainly related to crop growth and development even at low concentrations (Winkler et al. 2017). Note, however, due to their high costs, only a few kinds of oligosaccharides (such as the chitooligosaccharides) are currently used in agriculture.
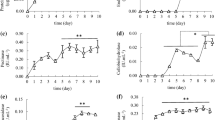
The function of synthesized COS was determined by evaluating their effect on plant growth using tomato as a model plant. Tomato seedlings were soaked with different concentrations of the COS prepared from CCR. On 6, 8, 10, and 12 days in the seedling stage, COS was sprayed for four times on the leaves of tomato. The COS solutions at 40, 80, and 160 mg/L concentration level could all significantly promote the growth of tomato seedlings, with 80 mg/L presenting the highest rate of promotion (Fig. 4A). It was also discovered that, spraying 40, 80, and 160 mg/L of COS (particularly 80 mg/L) on the leaves could promote the root development (Fig. 4B). This was similar to that observed previously for other oligosaccharides (Kananont et al. 2010) and suggested that the CCR-derived COS as prepared by sequential degradation and synthesis were functional and could play an important role in promoting growth of tomato, and likely other crops, as well.
Discussion
COS is one of the many oligosaccharides that have been documented to be useful in a variety of industries. Due to its wide application potential, in recent years, there have already been many endeavors trying to produce COS from either polymeric agricultural residues (Barbosa et al. 2020; Kendrick et al. 2022; Zhong et al. 2023) or simple sugars (such as glucose and cellobiose) (Schwaiger and Nidetzky 2022; Schwaiger et al. 2022; Zhong et al. 2020). Cellulase-catalyzed depolymerization is the solution for the agricultural residue feedstocks. However, because of the complexity and recalcitrance of polymeric lignocellulose, this process has to be assisted by the use of additional agents (e.g. ionic liquids) (Zhong et al. 2023) or pretreatment measures (e.g. phosphoric acid swelling) (Kendrick et al. 2022), which can be toxic to downstream application or increase the cost of production. Unlike other lignocellulosic agricultural residues, CCR is unique in that it contains mainly cellulose and lignin but nearly no xylan. However, even this decreased complexity did not allow efficient production of COS with any of the six selected candidate endo-glucanase, suggesting that the recalcitrance of crystalline cellulose and impediment by the lignin could be beyond the degrading ability of a single or simply combined selected endo-glucanases, even in presence of the Fenton reaction reagents with ability to break the lignin barrier. However, the possibility that other endo-glucanases work well with CCR cannot be excluded.
Bottom-up synthesis of COS from simple sugars does not have the disadvantages of using ionic liquids or requiring additional pretreatments. However, this route of synthesis starts only with glucose or simple sugars such as cellobiose. Although lignocellulosic biomass such as corncob residue cannot be directly used as the feedstock, we noted that these polymeric materials can be efficiently destructed by easily acquirable cellulase mixtures into glucose and simple sugars. Therefore, an alternative strategy, in parallel with endo-glucanase degradation, was examined by combining the use of a cellulase mixture to depolymerize cellulose and subsequently phosphorylases to synthesize COS from the produced simple sugars. Theoretically, the combinatorial strategy can integrate the merits from both routes and is particularly attractive for using the CCR, which has only cellulose and lignin left after extraction of xylan.
With this approach, CCR could be efficiently hydrolyzed by the T. reesei cellulase to glucose and cellobiose, which were then used to generate up to 100.3 g/L of COS with the degrees of polymerization of COS ranging from G2 to G6 using the combination of BaSP, CuCbP, and CcCdP. The highest yield of COS (up to 125 g/L from 36 g/L of glucose) was reported by Schwaiger et al (2022), when glucose was used as the starting sugar and E. coli expressing the phosphorylases were used as the whole cell catalysts. In this study 100.3 g/L of COS could be produced from 28 g/L of glucose and 3 g/L of cellobiose which were derived from an agricultural residue CCR. The reasons for the slightly lower yield could be as the following: (1) As CCR is composed of cellulose and lignin, it is possible that there are still agents that could slightly interfere with the phosphorylation reactions. (2) The ratio of glucose and cellobiose in cellulase-hydrolyzed CCR might not be optimal. (3) The amounts of enzymes and ratio of phosphorylases might also not be optimal. Pretreatment of the CCR hydrolysates such as using adsorbents and adjusting the amounts and glucose/cellobiose and phosphorylase ratios may assist in improving the transformation efficiency and yield of the COS in the future.
In this study, rather than focusing on only one cellodextrin phosphorylase to synthesize COS, we determined the behaviors of three different kinds of cellodextrin phosphorylases (OCP2, CcCdP, and TaCDP). TaCDP was active in catalyzing phosphorylation; however, the generated oligosaccharides were not the expected COS but most likely their isomeric forms. OCP2 and CcCdP gave rise to bona fide COS, albeit with much different patterns in oligomerization. Although we only determined the effect of CcCdP-derived COS on tomato growth, it is not unlikely that the two other oligosaccharides generated by OCP2 and TaCDP could have somewhat similar effects on growth of the plant. COS can also promote growth of probiotics bacteria. However, we noted that some gut bacteria (such as the gut bacteria Roseburia intestinalis and Bacteroides ovatus) can respond very differently to the same kind of oligosaccharides with varying sugar chain lengths (Leth et al. 2018). Therefore, as gut microbiota with different compositions are normally associated with specific healthy status of human and animals, the three COS obtained, if used as prebiotics, theoretically could provide more diversification to the gut microbiota. It could also be expected that with other cellodextrin phosphorylases, more COS profiles could be obtained.
Currently, several oligosaccharides with different glycosidic linkages, such as the alginate-derived oligosaccharides (Natsume et al. 1994), xyloglucan oligosaccharides (Cutillas-Iturralde and Lorences 1997), chito-oligosaccharides (Mukhtar Ahmed et al. 2020), pectate oligosaccharides (Iwasaki and Matsubara 2000), and xylo-oligosaccharides (Katapodis et al. 2002), have been proven to be able to promote plant growth. Herein, we demonstrated that COS can have a similar effect on promoting growth of a plant using tomato as a model. Therefore, the COS produced was functional as being able to stimulate growth of tomato, hinting its application potential in the crop and other industries. Thus, the use of COS can be an extra and new option in selecting the oligosaccharide-based plant growth promoter. CCR is the remnant of the corn cob with the xylan extracted and our study provides an approach to make more thorough utilization of this agricultural waste. In this study, the phosphorylases were expressed in E. coli, but using purified enzymes could raise the production cost. Surface display of these phosphorylases in E. coli, or express thermophlic phosphorylases (which would enable synthesis of COS at high temperatures without the need of pretreatments to break the E. coli cell wall), could help to reduce the cost in production. Alternatively, in order to reduce the cost, the phosphorylases can also be expressed as a secretory form in major industrial microbial workhorses such as P. pastoris, T. reesei, and Aspergillus niger. The studies to make functional surface-displayed phosphorylases and produce secretory enzymes in other microbial cells are in progress in our lab.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Amore A, Giacobbe S, Faraco V (2013) Regulation of cellulase and hemicellulase gene expression in fungi. Curr Genomics 14(4):230–249. https://doi.org/10.2174/1389202911314040002
Anasontzis GE, Lebrun MH, Haon M, Champion C, Kohler A, Lenfant N, Martin F, O’Connell RJ, Riley R, Grigoriev IV, Henrissat B, Berrin JG, Rosso MN (2019) Broad-specificity GH131 β-glucanases are a hallmark of fungi and oomycetes that colonize plants. Environ Microbiol 21(8):2724–2739. https://doi.org/10.1111/1462-2920.14596
Ávila PF, Silva MF, Martins M, Goldbeck R (2021) Cello-oligosaccharides production from lignocellulosic biomass and their emerging prebiotic applications. World J Microbiol Biotechnol 37(5):73. https://doi.org/10.1007/s11274-021-03041-2
Barbosa FC, Martins M, Brenelli LB, Ferrari FA, Forte MBS, Rabelo SC, Franco TT, Goldbeck R (2020) Screening of potential endoglucanases, hydrolysis conditions and different sugarcane straws pretreatments for cello-oligosaccharides production. Bioresour Technol 316:123918. https://doi.org/10.1016/j.biortech.2020.123918
Belaich A, Parsiegla G, Gal L, Villard C, Haser R, Belaich J-P (2002) Cel9M, a new family 9 cellulase of the Clostridium cellulolyticum cellulosome. J Bacteriol 184(5):1378–1384. https://doi.org/10.1128/JB.184.5.1378-1384.2002
Berto GL, Velasco J, Tasso Cabos Ribeiro C, Zanphorlin LM, Noronha Domingues M, Tyago Murakami M, Polikarpov I, de Oliveira LC, Ferraz A, Segato F (2019) Functional characterization and comparative analysis of two heterologous endoglucanases from diverging subfamilies of glycosyl hydrolase family 45. Enzyme Microb Technol 120:23–35. https://doi.org/10.1016/j.enzmictec.2018.09.005
Cangiano LR, Yohe TT, Steele MA, Renaud DL (2020) Invited review: strategic use of microbial-based probiotics and prebiotics in dairy calf rearing. Appl Anim Sci 36(5):630–651. https://doi.org/10.15232/aas.2020-02049
Cerdobbel A, De Winter K, Desmet T, Soetaert W (2010) Sucrose phosphorylase as cross-linked enzyme aggregate: improved thermal stability for industrial applications. Biotechnol J 5(11):1192–1197. https://doi.org/10.1002/biot.201000202
Choudhary RC, Kumaraswamy RV, Kumari S, Sharma SS, Pal A, Raliya R, Biswas P, Saharan V (2017) Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci Rep 7:11. https://doi.org/10.1038/s41598-017-08571-0
Cutillas-Iturralde A, Lorences EP (1997) Effect of xyloglucan oligosaccharides on growth, viscoelastic properties, and long-term extension of pea shoots. Plant Physiol 113(1):103–109. https://doi.org/10.1104/pp.113.1.103
De Groeve MRM, De Baere M, Hoflack L, Desmet T, Vandamme EJ, Soetaert W (2009) Creating lactose phosphorylase enzymes by directed evolution of cellobiose phosphorylase. Protein Eng Des Sel 22(7):393–399. https://doi.org/10.1093/protein/gzp017
De Winter K, Cerdobbel A, Soetaert W, Desmet T (2011) Operational stability of immobilized sucrose phosphorylase: continuous production of α-glucose-1-phosphate at elevated temperatures. Process Biochem 46(10):2074–2078. https://doi.org/10.1016/j.procbio.2011.08.002
Doiseau A-C, Rataboul F, Burel L, Essayem N (2014) Synergy effect between solid acid catalysts and concentrated carboxylic acids solutions for efficient furfural production from xylose. Catal Today 226:176–184. https://doi.org/10.1016/j.cattod.2013.10.034
Gao F, Hao Z, Sun X, Qin L, Zhao T, Liu W, Luo H, Yao B, Su X (2018) A versatile system for fast screening and isolation of Trichoderma reesei cellulase hyperproducers based on DsRed and fluorescence-assisted cell sorting. Biotechnol Biofuels 11(1):261. https://doi.org/10.1186/s13068-018-1264-z
Han J, Cao R, Zhou X, Xu Y (2020) An integrated biorefinery process for adding values to corncob in co-production of xylooligosaccharides and glucose starting from pretreatment with gluconic acid. Bioresour Technol 307:123200. https://doi.org/10.1016/j.biortech.2020.123200
Haq Iu, Akram F, Khan MA, Hussain Z, Nawaz A, Iqbal K, Shah AJ (2015) CenC, a multidomain thermostable GH9 processive endoglucanase from Clostridium thermocellum: cloning, characterization and saccharification studies. World J Microbiol Biotechnol 31(11):1699–1710. https://doi.org/10.1007/s11274-015-1920-4
Herr D (1980) Conversion of cellulose to glucose with cellulase of Trichoderma viride ITCC-1433. Biotechnol Bioeng 22:1610–1612. https://doi.org/10.1002/bit.260220806
Iwasaki K-I, Matsubara Y (2000) Purification of pectate oligosaccharides showing root-growth-promoting activity in lettuce using ultrafiltration and nanofiltration membranes. J Biosci Bioeng 89(5):495–497. https://doi.org/10.1016/S1389-1723(00)89104-5
Jiao LF, Song ZH, Ke YL, Xiao K, Hu CH, Shi B (2014) Cello-oligosaccharide influences intestinal microflora, mucosal architecture and nutrient transport in weaned pigs. Anim Feed Sci Technol 195:85–91. https://doi.org/10.1016/j.anifeedsci.2014.05.014
Kadowaki MAS, Camilo CM, Muniz AB, Polikarpov I (2015) Functional characterization and low-resolution structure of an endoglucanase Cel45A from the filamentous fungus Neurospora crassa OR74A: thermostable enzyme with high activity toward lichenan and β-glucan. Mol Biotechnol 57(6):574–588. https://doi.org/10.1007/s12033-015-9851-8
Kananont N, Pichyangkura R, Chanprame S, Chadchawan S, Limpanavech P (2010) Chitosan specificity for the in vitro seed germination of two Dendrobium orchids (Asparagales: Orchidaceae). Sci Hortic 124:239–247. https://doi.org/10.1016/j.scienta.2009.11.019
Karnaouri A, Matsakas L, Krikigianni E, Rova U, Christakopoulos P (2019) Valorization of waste forest biomass toward the production of cello-oligosaccharides with potential prebiotic activity by utilizing customized enzyme cocktails. Biotechnol Biofuels 12(1):285. https://doi.org/10.1186/s13068-019-1628-z
Katapodis P, Kavarnou A, Kintzios S, Pistola E, Kekos D, Macris BJ, Christakopoulos P (2002) Production of acidic xylo-oligosaccharides by a family 10 endoxylanase from Thermoascus aurantiacus and use as plant growth regulators. Biotechnol Lett 24(17):1413–1416. https://doi.org/10.1023/A:1019898414801
Kendrick EG, Bhatia R, Barbosa FC, Goldbeck R, Gallagher JA, Leak DJ (2022) Enzymatic generation of short chain cello-oligosaccharides from Miscanthus using different pretreatments. Bioresour Technol 358:127399. https://doi.org/10.1016/j.biortech.2022.127399
Leth ML, Ejby M, Workman C, Ewald DA, Pedersen SS, Sternberg C, Bahl MI, Licht TR, Aachmann FL, Westereng B, Abou Hachem M (2018) Differential bacterial capture and transport preferences facilitate co-growth on dietary xylan in the human gut. Nat Microbiol 3(5):570–580. https://doi.org/10.1038/s41564-018-0132-8
Li H, Deng A, Ren J, Liu C, Lu Q, Zhong L, Peng F, Sun R (2014) Catalytic hydrothermal pretreatment of corncob into xylose and furfural via solid acid catalyst. Bioresour Technol 158:313–320. https://doi.org/10.1016/j.biortech.2014.02.059
Liu G, Wei X, Qin Y, Qu Y (2010) Characterization of the endoglucanase and glucomannanase activities of a glycoside hydrolase family 45 protein from Penicillium decumbens 114–2. J Gen Appl Microbiol 56(3):223–229. https://doi.org/10.2323/jgam.56.223
Mirza O, Skov LK, Sprogøe D, van den Broek LAM, Beldman G, Kastrup JS, Gajhede M (2006) Structural rearrangements of sucrose phosphorylase from Bifidobacterium adolescentis during sucrose conversion. J Biol Chem 281(46):35576–35584. https://doi.org/10.1074/jbc.M605611200
Mukhtar Ahmed KB, Khan MMA, Siddiqui H, Jahan A (2020) Chitosan and its oligosaccharides, a promising option for sustainable crop production- a review. Carbohydr Polym 227:115331. https://doi.org/10.1016/j.carbpol.2019.115331
Natsume M, Kamo Y, Hirayama M, Adachi T (1994) Isolation and characterization of alginate-derived oligosaccharides with root growth-promoting activities. Carbohydr Res 258:187–197. https://doi.org/10.1016/0008-6215(94)84085-7
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T (2006) The path forward for biofuels and biomaterials. Science (new York, NY) 311(5760):484–489. https://doi.org/10.1126/science.1114736
Sathiyabama M, Manikandan A (2016) Chitosan nanoparticle induced defense responses in fingermillet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohydr Polym 154:241–246. https://doi.org/10.1016/j.carbpol.2016.06.089
Schwaiger KN, Nidetzky B (2022) Continuous process technology for bottom-up synthesis of soluble cello-oligosaccharides by immobilized cells co-expressing three saccharide phosphorylases. Microb Cell Fact 21(1):265. https://doi.org/10.1186/s12934-022-01984-1
Schwaiger KN, Voit A, Dobiašová H, Luley C, Wiltschi B, Nidetzky B (2020) Plasmid design for tunable two-enzyme co-expression promotes whole-cell production of cellobiose. Biotechnol J 15(11):2000063. https://doi.org/10.1002/biot.202000063
Schwaiger KN, Voit A, Wiltschi B, Nidetzky B (2022) Engineering cascade biocatalysis in whole cells for bottom-up synthesis of cello-oligosaccharides: flux control over three enzymatic steps enables soluble production. Microb Cell Fact 21(1):61. https://doi.org/10.1186/s12934-022-01781-w
Sheng T, Zhao L, Gao L, Liu W, Cui M, Guo Z, Ma X, Ho S, Wang A (2016) Lignocellulosic saccharification by a newly isolated bacterium, Ruminiclostridium thermocellum M3 and cellular cellulase activities for high ratio of glucose to cellobiose. Biotechnol Biofuels 9:172. https://doi.org/10.1186/s13068-016-0585-z
Su X, Mackie Roderick I, Cann Isaac KO (2012) Biochemical and mutational analyses of a multidomain cellulase/mannanase from Caldicellulosiruptor bescii. Appl Environ Microbiol 78(7):2230–2240. https://doi.org/10.1128/AEM.06814-11
Sun J, Zhang Z, Xue W, Zhou R, Wang Y, Wang Y (2022) Comparative study on preparation methods of xylooligosaccharides from corncob. China Condiment 47(06):192–195. https://doi.org/10.3969/j.issn.1000-9973.2022.06.036
Ubiparip Z, Moreno DS, Beerens K, Desmet T (2020) Engineering of cellobiose phosphorylase for the defined synthesis of cellotriose. Appl Microbiol Biotechnol 104(19):8327–8337. https://doi.org/10.1007/s00253-020-10820-8
Uyeno Y, Shigemori S, Shimosato T (2015) Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ 30(2):126–132. https://doi.org/10.1264/jsme2.ME14176
Warner CD, Go RM, García-Salinas C, Ford C, Reilly PJ (2011) Kinetic characterization of a glycoside hydrolase family 44 xyloglucanase/endoglucanase from Ruminococcus flavefaciens FD-1. Enzyme Microb Technol 48(1):27–32. https://doi.org/10.1016/j.enzmictec.2010.08.009
Westereng B, Arntzen MØ, Aachmann FL, Várnai A, Eijsink VGH, Agger JW (2016) Simultaneous analysis of C1 and C4 oxidized oligosaccharides, the products of lytic polysaccharide monooxygenases acting on cellulose. J Chromatogr A 1445:46–54. https://doi.org/10.1016/j.chroma.2016.03.064
Winkler AJ, Dominguez-Nunez JA, Aranaz I, Poza-Carrion C, Ramonell K, Somerville S, Berrocal-Lobo M (2017) Short-chain chitin oligomers: promoters of plant growth. Mar Drugs 15(2):21. https://doi.org/10.3390/md15020040
Wu Y, Mao G, Fan H, Song A, Zhang Y-HP, Chen H (2017) Biochemical properties of GH94 cellodextrin phosphorylase THA_1941 from a thermophilic eubacterium Thermosipho africanus TCF52B with cellobiose phosphorylase activity. Sci Rep 7(1):4849. https://doi.org/10.1038/s41598-017-05289-x
Yang W, Ajapur V, Krishnamurthy K, Feng H, Yang R, Rababah T, Yang C, Hao F, Ruijin Y (2009) Expedited extraction of xylan from corncob by power ultrasound. Int J Agric Biol Eng 2:76–83. https://doi.org/10.3965/j.issn.1934-6344.2009.04.076-083
Yang H, Shi P, Liu Y, Xia W, Wang X, Cao H, Ma R, Luo H, Bai Y, Yao B (2017) Loop 3 of fungal endoglucanases of glycoside hydrolase family 12 modulates catalytic efficiency. Appl Environ Microbiol 83(6):e03123-e3216. https://doi.org/10.1128/AEM.03123-16
Yi Z, Su X, Revindran V, Mackie R, Cann I (2013) Molecular and biochemical analyses of CbCel9A/Cel48A, a highly secreted multi-modular cellulase by Caldicellulosiruptor bescii during growth on crystalline cellulose. PLoS One 8:e84172. https://doi.org/10.1371/journal.pone.0084172
Zhang Y-HP, Ding S-Y, Mielenz JR, Cui J-B, Elander RT, Laser M, Himmel ME, McMillan JR, Lynd LR (2007) Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol Bioeng 97(2):214–223. https://doi.org/10.1002/bit.21386
Zhao XM, She XP, Du YG, Liang XM (2007) Induction of antiviral resistance and stimulary effect by oligochitosan in tobacco. Pestic Biochem Physiol 87(1):78–84. https://doi.org/10.1016/j.pestbp.2006.06.006
Zhong C, Nidetzky B (2020) Three-enzyme phosphorylase cascade for integrated production of short-chain cellodextrins. Biotechnol J 15(3):e1900349. https://doi.org/10.1002/biot.201900349
Zhong C, Luley-Goedl C, Nidetzky B (2019) Product solubility control in cellooligosaccharide production by coupled cellobiose and cellodextrin phosphorylase. Biotechnol Bioeng 116(9):2146–2155. https://doi.org/10.1002/bit.27008
Zhong C, Ukowitz C, Domig KJ, Nidetzky B (2020) Short-chain cello-oligosaccharides: intensification and scale-up of their enzymatic production and selective growth promotion among probiotic bacteria. J Agric Food Chem 68(32):8557–8567. https://doi.org/10.1021/acs.jafc.0c02660
Zhong C, Zajki-Zechmeister K, Nidetzky B (2023) Effect of ionic liquid on the enzymatic synthesis of cello-oligosaccharides and their assembly into cellulose materials. Carbohydr Polym 301:120302. https://doi.org/10.1016/j.carbpol.2022.120302
Funding
This research was supported by the National Key R&D Program of China (2022YFA0912301 and 2021YFC2100402) and the China Agriculture Research System of MOF and MARA (CARS-41).
Author information
Authors and Affiliations
Contributions
YL, XS, and HH conceived and designed research. XS, HH, and BY provided funding. YL conducted the experiments and analyzed the data. WJ, XS, ZH, and XW supervised the experiments. YW, WZ, YB, XQ, and HL analyzed data. YL wrote the original manuscript. XS edited the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Y., Ji, W., Sun, X. et al. Production of cello-oligosaccharides from corncob residue by degradation-synthesis reactions. Appl Microbiol Biotechnol 108, 13 (2024). https://doi.org/10.1007/s00253-023-12832-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12832-6