Abstract
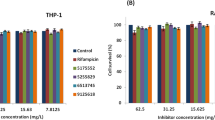
PK34 is a D29 mycobacteriophage-derived anti-microbial peptide (AMP) with anti-Mycobacterium tuberculosis activity. It is expected to become an auxiliary drug for the treatment of M. tuberculosis infection, or as a template for the development of anti-M. tuberculosis drugs. The focus of this paper is to obtain recombinant PK34 by a novel method of prokaryotic expression and purification by affinity chromatography. The minimum inhibitory concentration (MIC) of recombinant PK34 was better than that of synthetic PK34 as measured by the microplate-based Alamar Blue assay (MABA). In order to further compare the different anti-bacterial effects of PK34 obtained by the two methods on M. tuberculosis, the bacterial changes after drug incubation were observed at the microscopic level by transmission electron microscopy (TEM). In order to apply PK34 to clinical treatment earlier in the future, this paper tested the maximum non-toxic concentration of recombinant PK34 to the two most studied immune cells, RAW264.7 and THP-1, through cytotoxicity experiments. The maximum non-toxic concentration was the same as the MIC of recombinant PK34 to M. tuberculosis H37Rv, and both were 12.5 μg/mL. The monoclonal antibodies against PK34 and their hybridoma cell lines were prepared using recombinant PK34 as the antigen. Next, we obtained the gene sequence of the monoclonal antibody, which was prepared for the basic research of PK34 in M. tuberculosis treatment. In addition, the possible molecular docking mode between PK34 and trehalose-6,6-dimycolate (TDM) was predicted by AI simulation. To sum up, this paper provides a new idea for the birth of more new AMPs of the same type as PK34 in the future.
Key points
• Design and prepare a novel recombinant PK34 anti-microbial peptide.
• Recombinant PK34 has higher purity and anti-bacterial activity than synthetic PK34.
• The monoclonal antibody against recombinant PK34 was prepared and sequenced.





Similar content being viewed by others
Data availability
The data sets used for the current study are available from the corresponding author upon reasonable request.
References
Al-Humadi HW, Al-Saigh RJ, Al-Humadi AW (2017) Addressing the challenges of tuberculosis: a brief historical account. Front Pharmacol 8:689. https://doi.org/10.3389/fphar.2017.00689
Arranz-Trullén J, Lu L, Pulido D, Bhakta S, Boix E (2017) Host antimicrobial peptides: the promise of new treatment strategies against tuberculosis. Front Immunol 8:1499. https://doi.org/10.3389/fimmu.2017.01499
Bhunia A, Mohanram H, Bhattacharjya S (2009) Lipopolysaccharide bound structures of the active fragments offowlicidin-1, a cathelicidin family of antimicrobial and antiendotoxic peptide from chicken, determined by transferrednuclear overhauser effect spectroscopy. Biopolymers 92(1):9–22. https://doi.org/10.1002/bip.21104
Cunningham AF, Spreadbury CL (1998) Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J Bacteriol 180(4):801–808. https://doi.org/10.1128/JB.180.4.801-808.1998
Jeżowska-Bojczuk M, Stokowa-Sołtys K (2018) Peptides having antimicrobial activity and their complexes with transition metal ions. Eur J Med Chem 143:997–1009. https://doi.org/10.1016/j.ejmech.2017.11.086
Johnson ES (2004) Protein modification by SUMO. Annu Rev Biochem 73:355–382. https://doi.org/10.1146/annurev.biochem.73.011303.074118
Khara JS, Wang Y, Ke XY, Liu S, Newton SM, Langford PR, Yang YY, Ee PL (2014) Anti–mycobacterial activities of synthetic cationic alpha–helical peptides and their synergism with rifampicin. Biomaterials 35:2032–2038. https://doi.org/10.1016/j.biomaterials.2013.11.035
Li MX, Luo SP, Zhang YF, Jia LN, Yang CY, Peng XX, Zhao RL (2020) Production, characterization, and application of a monoclonal antibody specific for the extracellular domain of human P2X7R. Appl Microbiol Biotechnol 104(5):2017–2028. https://doi.org/10.1007/s00253-019-10340-0
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25(12):677–686. https://doi.org/10.1016/j.it.2004.09.015
Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13. https://doi.org/10.12703/P6-13
Malakhov MP, Mattern MR, Malakhova OA, Drinker M, Weeks SD, Butt TR (2004) SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J Struct Funct Genomics 5(1–2):75–86. https://doi.org/10.1023/B:JSFG.0000029237.70316.52
Marblestone JG, Edavetta SC, Lim Y, Lim P, Zuo X, Butt TR (2006) Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci 15(1):182–189. https://doi.org/10.1110/ps.051812706
Oliveira GS, Costa RP, Gomes P, Gomes MS, Silva T, Teixeira C (2021) Antimicrobial peptides as potential anti-tubercular leads: a concise review. Pharmaceuticals 14:323. https://doi.org/10.3390/ph14040323
Peroutka IR, Orcutt SJ, Strickler JE, Butt TR (2011) SUMO fusion technology for enhanced protein expression and purification in prokaryotes and eukaryotes. Methods Mol Biol 705:15–30. https://doi.org/10.1007/978-1-61737-967-3_2
Rybniker J, Kramme S, Small PL (2006) Host range of 14 mycobacteriophages in Mycobacterium ulcerans and seven other mycobacteria including Mycobacterium tuberculosis–application for identification and susceptibility testing. J Med Microbiol 55:37–42. https://doi.org/10.1099/jmm.0.46238-0
Su ZJ, Huang YD, Zhou QN, Wu ZL, Wu XP, Zheng Q, Ding CC, Li XK (2006) High-level expression and purification of human epidermal growth factor with SUMO fusion in Escherichia coli. Protein Pept Lett 13(8):785–792. https://doi.org/10.2174/092986606777841280
Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A (2011) Immunological biomarkers of tuberculosis. Nat Rev Immunol 11:343–354. https://doi.org/10.1038/nri2960
Wang HY, Xiao YC, Fu LJ, Zhao HX, Zhang YF, Wan XS, Qin YX, Huang YD, Gao HC, Li XK (2010) High-level expression and purification of soluble recombinant FGF21 protein by SUMO fusion in Escherichia coli. BMC Biotechnol 10:14. https://doi.org/10.1186/1472-6750-10-14
Wang M, Lin JL, Sun QL, Zheng KW, Ma Y, Wang JF (2019) Design, expression, and characterization of a novel cecropin A-derived peptide with high antibacterial activity. Appl Microbiol Biotechnol 103:1765–1775. https://doi.org/10.1007/s00253-018-09592-z
Wei L, Gao J, Zhang S, Wu S, Xie Z, Ling G, Kuang YQ, Yang Y, Yu H, Wang Y (2015) Identification and characterization of the first cathelicidin from sea snakes with potent antimicrobial and anti–inflammatory activity and special mechanism. J Biol Chem 290:16633–16652. https://doi.org/10.1074/jbc.M115.642645
Wei L, Wu J, Liu H, Yang H, Rong M, Li D, Zhang P, Han J, Lai R (2013) A mycobacteriophage-derived trehalose-6,6-dimycolate-binding peptide containing both antimycobacterial and anti-inflammatory abilities. FASEB J 27(8):3067–3077. https://doi.org/10.1096/fj.13-227454
Wu M, Zhao H, Li M, Yue Y, Xiong S, Xu W (2017) Intranasal vaccination with mannosylated chitosan formulated DNA vaccine enables robust IgA and cellular response induction in the lungs of mice and improves protection against pulmonary mycobacterial challenge. Front Cell Infect Microbiol 7:445–445. https://doi.org/10.3389/fcimb.2017.00445
Wu XP, Nie CJ, Huang ZF, Nie YF, Yan QX, Xiao YC, Su ZJ, Huang YD, Xiao J, Zeng YY, Tian Y, Feng WK, Li XK (2009) Expression and purification of human keratinocyte growth factor 2 by fusion with SUMO. Mol Biotechnol 42(1):68–74. https://doi.org/10.1007/s12033-008-9135-7
Yang Y, Liu Z, He X, Yang J, Wu J, Yang H, Li M, Qian Q, Lai R, Xu W, Wei L (2019) A small mycobacteriophage-derived peptide and its improved isomer restrict mycobacterial infection via dual mycobactericidal-immunoregulatory activities. J Biol Chem 294:7615–7631. https://doi.org/10.1074/jbc.RA118.006968
Acknowledgements
The authors would like to thank the faculty and staff of the Specialized lab for medical examination of Weifang Medical University.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81802054 and 81902170). Support program for Youth Innovation Technology in Colleges and Universities of Shandong Province of China (Grant No. 2021KJ106). The Tai-Shan Scholar Program from Shandong Province (Grant No. tsqn202103116).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: QL and JQW; performed the experiments: JQW, TXY, and XYH; analyzed the data: QL, JQW, HL, and TXY; prepared figures and/or tables: JQW and TXY; drafted manuscripts: QL, JQW, ZJY, and WG; and revised the manuscript: QL, JQW, ZJY, and WG. All the authors approved the submitted manuscript.
Corresponding author
Ethics declarations
Ethical approval
All the experiments involving mice were performed in accordance with the Chinese National Laboratory Animal-Guideline for Ethical Review of Animal Welfare and approved by the Institutional Animal Care and Use Committee of Weifang Medical University. All the applicable international, national, and institutional guidelines for the care and use of animals were strictly followed. The study was approved by the Ethics Committee of Weifang Medical University, and all the research methods were in compliance with the relevant regulations and guidelines.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Yuan, T., He, X. et al. Production, characterization, and application of phage-derived PK34 recombinant anti-microbial peptide. Appl Microbiol Biotechnol 107, 163–174 (2023). https://doi.org/10.1007/s00253-022-12306-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12306-1