Abstract
The need for biosensors has evolved in the detection of molecules, diseases, and pollution from various sources. This requirement has headed to the development of accurate and powerful equipment for analysis using biological sensing component as a biosensor. Biosensors have the advantage of rapid detection that can beat the conventional methods for the detection of the same molecules. Bio-chemiluminescence-based sensors are very sensitive during use in biological immune assay systems. Optical biosensors are emerging with time as they have the advantage that they act with a change in the refractive index. Carbon nanotube-based sensors are another area that has an important role in the biosensor field. Bioluminescence gives much higher quantum yields than classical chemiluminescence. Electro-generated bioluminescence has the advantage of miniature size and can produce a high signal-to-noise ratio and the controlled emission. Recent advances in biological techniques and instrumentation involving fluorescence tag to nanomaterials have increased the sensitivity limit of biosensors. Integrated approaches provided a better perspective for developing specific and sensitive biosensors with high regenerative potentials. This paper mainly focuses on sensors that are important for the detection of multiple molecules related to clinical and environmental applications.
Key points
• The review focusses on the applications of luminescence-based, surface plasmon resonance-based, carbon nanotube-based, and graphene-based biosensors
• Potential clinical, environmental, agricultural, and food industry applications/uses of biosensors have been critically reviewed
• The current limitations in this field are discussed, as well as the prospects for future advancement
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
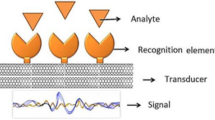
Biosensors have rapidly emerged as a more straightforward, faster, and convenient way for the detection of molecules, diseases, and pollution from various sources. Biosensors have the advantage of rapid detection, specificity, low reaction time, and high output that could beat the conventional methods for the detection of the same molecules (Fig. 1). Humans have exploited the environment for their selfish purposes that lead to many global problems such as global warming, water pollution, and solid waste pollution apart from pollutions; biosensors are developed in various fields that detect various deadly diseases such as cancer. This paper mainly focuses on sensors that are important for the detection of multiple molecules related to clinical and environmental applications. Different types of biosensors are disclosed and characterized. Advances in developed biosensors as well as their future potential applications are discussed. The review summarizes novel developed biosensors and however also highlights notable earlier-developed biosensors. The main purpose of this review is to provide an overview in the rapid developing area of modern detectors and their possible utilization in the future.
Chemiluminescence-based sensors are mainly of three types: (1) bio-chemiluminescence-based, (2) thermo-chemiluminescence-based, and (3) electrochemiluminescence (Roda and Guardigli 2012). Bio-chemiluminescence-based sensors are very sensitive when they are used in biological immune assay systems; they can detect up to attomole concentrations. Chemiluminescence technology is used widely for biologically important molecules (Roda et al. 2016). Discussing clinical applications, breast cancer is a major issue, and there are so many techniques being used, MRI mammography and some immunological techniques such as enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA), but these techniques have some drawbacks such as they are time-consuming and sometimes give false-positive results; to avoid this, so many biosensors are being developed which provide a signal when a particular biomarker related to breast cancer is detected in a sample (Mittal et al. 2017). Surface plasmon resonance is a widely used immunological technique based on antibody-based detection of molecules. Gold and other noble metal-based biosensors have come into existence recently; they use the differential properties of gold nanoparticles to enhance the detection, as because of the size and shape, the nanoparticle gives distinct signals so that it can be used for the detection of different clinically important molecules (Cao et al. 2014). Gold nanoparticle-based SPR biosensor has been developed, which can detect hepatitis B infection in very low concentrations in Tris buffer-based systems (Wang et al. 2010).
These are successful technologies; however, there are so many chemiluminescence-based sensors that have not been developed for the detection of molecules but are not available commercially in the market (Park and Kricka 2014). Biosensor research aims to produce low-cost semi-quantitative and fast biosensors, which can work virtually in all kinds of environments and are relatively easy, not consisting of many economic steps (Marquette and Blum 2010). The development in chemiluminescence and bioluminescence is getting fused, and it must be to develop small-sized sensing devices and new molecules that can help in the development of the sensors for personalized diagnostics (Roda and Guardigli 2012).
Optical biosensors are emerging with time as they have the advantage that they act with a change in the refractive index. These biosensors have an additional benefit as they do not get interference from magnetic fields. These sensors are useful for a wide range of applications, from diagnostics to the battlefield. There are mainly two kinds of strategy: first is to label the target molecule with the fluorescent tag so that upon binding it will release signal and it has very high sensitivity only with a disadvantage that the labelling cannot be controlled properly. The second is direct sensing in which binding of the molecule with the target will lead to a change in the refractive index that is very sensitive in comparison to fluorescent-based labelling. There are some differences between the direct detection systems and fluorescent-based systems; however, both technologies are being used for sensing for a wide area of applications as they provide good sensitivity and can determine analyte concentration very efficiently (Fan et al. 2008).
Carbon nanotube-based sensors are another area that has an important role in the biosensor field. Carbon nanotubes are being used vastly in biosensing because they have some unique properties. Carbon nanotubes may be single-walled or multi-walled and mostly composed of rolled graphene sheets. Carbon nanotubes can act as semiconductors or superconductors according to needs and modifications. Carbon nanotubes have been successfully used in enzyme-linked sensing mostly, and they can be used for sensing with redox reaction coupled enzymes as well as for the detection of glucose using redox mechanisms. Apart from the enzymes, it can also be coupled with antibodies or DNA molecules for selective binding applications (Wang 2005).
Chemiluminescence fluorescence technologies require the detection of photons emitted by the system for that various technologies are being used for detection starting from photomultiplier tubes to cell-phone-based detection of the emitted light in recent days. Photomultiplier tubes have some disadvantage as the detection is between 360 and 670 nm. Apart from the detection range, it consumes high electricity. However, recent flat photomultiplier tube has removed disadvantages and has been proven to be good for the detection of the emitted wavelength. Advanced detectors include charge-coupled devices (CCD), complementary metal-oxide semiconductors (CMOS), and silicon and organic photodiodes. These have very good sensitivity and have the advantage of small size, and they are also able to detect multiple spots at a time (Lengger et al. 2014; Zhou et al. 2014b; Zangheri et al. 2015). Recent back-illuminated CMOS have attracted great attention because of their low power consumption and high sensitivity.
CMOS offers advantages like a high-resolution and high signal-to-noise ratio, and pixel size is significantly reduced, which provides proper resolution even in low light conditions. Recently, large area CMOS was used for the successful detection of multiple chemiluminescence spots simultaneously (Bolton et al. 2002; Sandeau et al. 2015). Cooled CCDs are proven to give the best resolutions. Coloured CCDs are being developed which can be used with the combination of cell phones and tablets, which can detect luminescence with the device camera; they are proven good, but cooled CCDs are still gold standard (Roda et al. 2014b, 2014a). Thin-film photodiodes are another relatively inexpensive thing that can be used for the detection of amorphous silicon thin-film photodiodes, organic photodiodes, and carbon nanotubes coated with photovoltaic polymers (Shim and Ahn 2012; Caputo et al. 2013).
Types of biosensors
Several types of biosensors have been discussed, few are luminescence-based, some are surface plasmon resonance-based, some are optical, and some are carbon nanotube-based sensors. The present section will discuss some essential advances in biosensors. Figure 2 shows the types of biosensors that will be discussed in the following subsections.
Luminescence-based biosensors
There are different types of biosensor in which the luminescence is achieved by different principles; in this section, we will discuss a few of the luminescence-based biosensors.
Chemiluminescence
Chemiluminescent assays generally use direct labelling with the isoluminol and acridinium esters. The sensitivity can be up to nano-molar level, and signal-to-noise ratio is very high. Acridinium salts give very high chemiluminescence value in aqueous solutions with respect to isoluminol; however, the phenomenon is slow because acridinium salts react slowly with hydrogen peroxide because they are in a bound state with bases (Osman et al. 2000). Enzyme labelling is preferred over direct labelling as the enzyme increases the signal in comparison to direct labelling. Horseradish peroxidase (HRP) is generally used for the labelling; it converts luminol, which decays photon in the presence of hydrogen peroxide (Créton and Jaffe 2001). To enhance the sensitivity of these assays, electron transfer mediators such as indophenols, substituted phenols, N-alkyl phenothiazines, and substituted boronic acids apart from these 4-dialkylaminopyridine which is a nucleophilic acylation catalyst were also found to increase the intensity of the assay (Marzocchi et al. 2008; Zomer 2010). Apart from mentioned compounds, acridinium compounds can also be used with the horseradish peroxidase for the illumination assay (Osman et al. 2000). Recently, new hybrid molecules are being designed, which may replace the enzyme-based labels. Recently, DNAzymes are being synthesized, which can be designed to have HRP like activity, and the advantage of DNAzymes is that they have higher thermostability in comparison to classical enzyme-based labels. In addition to these molecules, nanoparticle-based systems have also been designed to enhance signal amplification (Huang et al. 2014a; Li et al. 2014; Park et al. 2015; Yu and He 2015).
Bioluminescence
Bioluminescence is a phenomenon that involves biological systems such as proteins and organisms. The advantage of bioluminescence is that it gives much higher quantum yields than classical chemiluminescence. Bioluminescence uses mostly the luciferase enzyme; in the presence of ATP, it gives the luminescence (Niwa et al. 2010; Thouand and Marks 2014). Recently, dihydrofolate reductase-based sensor has been developed for the detection of methotrexate. The protein is the receptor for the methotrexate, so it is used for sensing so that overdose of methotrexate can be prevented (Yu et al. 2017). Bioluminescence by luciferase requires ATP. This property has been exploited for decades to date for the development of various bioluminescence assays (Chappelle and Levin 1968; Borghei and Hall 2014). As the ATP concentration is proportional with the luminescence, luciferase-based bioassays are being used for the measurement of cell concentration, and ultrasensitive assays have been able to detect the ATP up to attomole level (Satoh et al. 2004). So many portable devices and kits are available in the market such as Milliflex® Rapid Testing (Merck-Millipore) and Clean-Trace™ (3 M™). The modification of luciferin and mutation in the luciferase can lead to tuneable biosensor such as mutation, and proper substrate modifications have led to the shift of emission towards red colour. Caged luciferins were used for the detection of small active biomolecules and live imagining studies (Hosseinkhani 2011; Li et al. 2013; Mofford et al. 2014).
Thermo-chemiluminescence
Thermo-chemiluminescence emits photons as a result of the thermolysis of a suitable molecule; it is usually a 1,2-dioxetane derivative, which leads to an exciting product. Adamantylideneadamantane 1,2-dioxetane derivatives were first used in 1980s as labels in immune-assays (Hummelen et al. 1986, 1988). A disadvantage with the thermo-chemiluminescence is that they require high temperature around 200–250 °C for detection; however, fluorescent additives were used to enhance the sensitivity, but the technique was not so useful. The advantage of the technique is that it doesn’t require the addition of any chemicals; recently, researchers tried to redevelop this technique; they used acridine-based systems to produce thermo-chemiluminescence around 80–100 °C (Roda and Guardigli 2012; Di Fusco et al. 2015).
Electro-generated chemiluminescence
Electro-generated bioluminescence is generated by the transfer of electrons at the electrode that leads to the emission of electro-generated photon chemiluminescence which can be controlled by the potential which is applied at the electrode; hence, the time and location of the photon emission can be controlled. This technique has the advantage of miniature size and can produce a high signal-to-noise ratio and the controlled emission. Newly developed sensors use inorganic metal complexes, and ruthenium-based sensors that have tiny size have been developed (Pyati and Richter 2007; Zhou et al. 2014a).
Surface plasmon resonance-based biosensors
Surface plasmon resonance (SPR) is a quite sensitive technique that provides a high signal-to-noise ratio with high specificity, and it doesn’t require labelling of any kind. Liedberg et al., in the 1980s, started the use of surface plasmon resonance for biosensing since then, and this has been used as one of the most sensitive techniques (Liedberg et al. 1983). Figure 3 shows the broad principle of SPR-based sensing. Surface plasmon wave (SPW) is generated by two different surfaces that have opposite dielectric signs: one is metal such as gold, and silver another one is dielectric. According to the excitation mechanism, there are several types of SPR-based sensing technologies prism coupling (Matsubara et al. 1988), grating coupling (Yu et al. 2004; Alleyne et al. 2007), waveguide coupling (Liedberg et al. 1993), and optical fibre coupling (Sharma et al. 2007).
The general principle of surface plasmon resonance. Polarize light is coupled by a glass prism on the biosensor chip. Thin-film of gold coating the chip is integrated with a flow channel for continuous flow of buffer. At a defined incidence angle, the surface plasmon resonance results in the decrease of the reflected light intensity, characteristic of the specific reflection angle. The shift of the reflection angle reveals a change in the composition of the medium near the thin gold layer as a result of the analyte binding to the biorecognition element (green and blue reflection intensity curve on the screen) (Brogioni and Berti 2014). Figure created with BioRender.com
In prism-based coupling, the incident light is reflected at the metal surface, and that has a resonant wavelength; however, the disadvantage with this method is the prism is quite bulky, and it is difficult to integrate; however, commercial instruments are available on this principle. Wave guide-based coupling works the same as prism-based, but the advantage is that waveguide is light and can be easily integrated with other electric components. In waveguide-based coupling, the light passes through the metal interphase and produces the SPW, which ultimately leads to detection. In the fibre optic-based coupling, the optical fibre is modified in various ways that allow the integration of surface with the fibre, and it provides excellent SPW. Grating-based technologies are very cheap, and they can be produced in bulk and provides the same results (Fan et al. 2008). Recently, aptamer-based SPR and bacterial detection sensors have been developed, which can detect targets in great sensitivity and complexity (Zhu et al. 2015; Vaisocherová-Lísalová et al. 2016).
Carbon nanotube-based biosensors
Carbon nanotubes (CNT) have been a great field of study since the time of their discovery. CNTs have great applications in the field of biosensing because they have properties like a high surface to volume ratio and they are ultrasensitive to the binding of the biological molecules and they can respond to the oxidation–reduction occurring on the surface coated molecules. CNTs can be used for the immobilization of enzymes because of the large surface area. They are commercially and economically feasible for large-scale productions as well. CNTs can be synthesized using several ways such as arc discharge laser ablation (Yang et al. 2015). Various biosensors have been developed based on CNTs; here, we will discuss a few. Enzyme-linked CNT-based biosensors are being developed for a wide range of applications. Glucose detection biosensors based on glucose oxidase have been developed as the detection of glucose is very important for diabetic individuals. Chitosan gel and CTNs based glucose biosensor have also been developed by researchers. Chitosan-based glucose biosensor was very robust and did not show interference with other substances like ascorbic acid in normal physiological conditions, and it had very high sensitivity (Fatoni et al. 2013; Pourasl et al. 2014). Apart from enzyme-based biosensors, a non-enzymatic glucose biosensor was also developed, which uses copper and nickel nanoparticles and electrode-based detection of the glucose. It is highly sensitive and low interference (Lin et al. 2013). Enzyme-linked CNT-based biosensors are mainly developed using four strategies, (1) covalent bonding, (2) crosslinking, (3) adsorption, and (4) embedding; using these techniques, so many sensors have been developed such as glucose oxidase, laccase, tyrosinase, and horseradish peroxidase (Yang et al. 2015). DNA-based CNTs biosensors have also been developed, which use DNA as the sensing molecule, and CNT acts as the base. Most of the CNT-based DNA sensors use single-stranded DNA rather than double-stranded DNA as it absorbs more rapidly on the surface of CNTs. Apart from this completely electronic CNT-based DNA sensors which have also been developed, CNT-based DNA sensors have also been developed for methylation regulation studies (Bachilo et al. 2002; Tang et al. 2006; Huang et al. 2014b). Figure 4 shows CNT functionalization mediated by covalent and noncovalent interactions.
Graphene-based biosensors
Pristine graphene, polycrystalline graphene, graphene oxide (GO), reduced graphene oxide (rGO), and graphene quantum dots (GQDs) are the different forms that are being used in the biosensor (Fig. 5). Pristine graphene is a flawless lattice of honeycomb-like structures of graphene with sp2 hybridization. Polycrystalline graphene is made with grain-like graphene crystal with defined boundaries. GQDs are tiny nanoparticle of graphene; generally, they have a diameter of 20 nm. Graphene oxide is also an sp2 hybridized lattice of carbon molecules that are disrupted by sp3 hybridized carbon molecules (Morales-Narváez et al. 2017). Heterogeneous electron transfer (HET) in reduced graphene oxide is because of their functional groups, the number of functional groups depends on the reduction method by which reduced graphene oxide is synthesized (El-Kady and Kaner 2013). Paper-based reduced graphene oxide biosensors have been developed for the detection of cancer (Wu et al. 2013). Graphene derivatives are responsive both in terms of fluorescence and quenching; however, these properties highly depend upon the layers of the graphene (Chen et al. 2010). Graphene-coupled single-stranded DNA-based sensors have been developed, which is quenched in the absence of analyte, and in the presence, it will start to fluoresce. Escherichia coli detection is also made possible up to 10 colony-forming units/mL in the sample using graphene-based fluorescent biosensors (Morales-Narváez et al. 2015).
Advances and potential applications of biosensors
Biosensors in agriculture and food industry
Various techniques are being used in agriculture for the determination of health status in crops including polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA), immunofluorescence (IF), gas chromatography-mass spectrometry (GC–MS), and flow cytometry (FCM). These methods allow us to recognize several diseases, nutrient deficiency, sensitivity to weather conditions, and stress conditions in plants. Presently, the stress condition in plants is being detected through changes in chemical indicator levels. However, the existing methods to analyse health condition in crops are time-consuming and laborious and developed for laboratory conditions. Advancements in biosensors seem to be crucial in a deeper understanding of agricultural processes (Kundu et al. 2019). In 2015, Larrieu et al. reported that a fluorescence-based biosensor to analyse the jasmonate signaling in plants has been developed. The biosensor provides information about hormone distribution in plant under abiotic and biotic stresses (Larrieu et al. 2015). Chong et al. also developed a fluorescence-based biosensor to detect early signs of water stress in plants before the permanent wilting point is reached (Chong et al. 2007).
Harvested fruits before shipment or distribution to consumers are in general stored and allowed to ripen for a certain period. During storing and ripening of the fruit, there are changes in the balance of chemical composition that are important to monitor. Simple monitoring systems ensure the required quality of the fruit for final consumers. A needle-type biosensor for the determination of sugars in fruits has been developed and applied for the analysis of glucose and sucrose concentrations in banana, orange, and kiwifruit. The biosensor combined three conventional electrodes (working, reference, and counter electrode), and side effects of citric and malic acids on both sucrose and glucose needle-type biosensors were evaluated to be small (Heineman et al. 2016). Dudchenko et al. developed a silicate/glucose oxidase-based biosensor for the determination of glucose in juices and nectars. A series of fruit juices and nectars were analysed using the biosensor, and the obtained results showed a good correlation (R = 0.99) with the data of high-performance liquid chromatography (HPLC). Even the biosensor sensitivity reached four- to fivefold higher levels compared to glucose oxidase-based biosensor (Dudchenko et al. 2016). An amperometric glutamate biosensor was developed and reported by Soldatkina et al. in 2017. The biosensor for glutamate detection uses a typical method of glutamate oxidase immobilization via adsorption on silicate particles. As an amperometric sensor, the disc platinum electrode is used. The linear range of glutamate detection was from 2.5 to 450 μM, and the limit of detection was 1 μM. The authors suggest the utilization of the developed biosensor in real samples with potential application in the food industry, fundamental and clinical medicine, neurophysiology, and neuropathology and in analytical biochemistry and biotechnology (Soldatkina et al. 2017). An electrochemical biosensor for detection of naringin, a flavonoid compound present in different citrus fruits, was reported by Ensafi et al. in 2016. The biosensor utilizes a modified pencil graphite electrode with a limit of detection of 10 ng/mL. Biosensing methodology is based on the interaction of naringin with DNA. Change in the oxidation signals of adenine and guanine was used as probes for the biosensor evaluation, using differential pulse voltammetry. The authors highlighted advantages of the proposed biosensor which include a wide linear dynamic range, low detection limit, ease of application, high speed, selectivity, and cost-effectiveness (Ensafi et al. 2016).
Further possible applications of biosensors in the agriculture and food industry result from requirements on food quality. Global agriculture nowadays unambiguously depends on the application of various chemicals, such as fertilizers, herbicides, and pesticides. Despite their positive effects on crops in the pre-harvest stage, the presence of chemicals in the post-harvest stage of crops is not required. The residues of agricultural chemicals represent a potential risk for consumers, causing several health problems, allergies, and anaphylaxis even death. Therefore, to avoid unintentional poisoning from agricultural chemicals, it is necessary to determine their levels in crops. Although many techniques involving HPLC, gas chromatography (GC), nuclear magnetic resonance (NMR), ultraviolet–visible spectroscopy (UV–Vis), and Fourier-transform infrared spectroscopy (FTIR) are advantageous and beneficial, demand for novel, fast, cheap, and universal detection systems remains a challenge (Kundu et al. 2019). The most frequently used pesticides to crop protection, due to their great efficiency, were organophosphorus pesticides (OPs). However, these compounds may cause environmental pollution and may threaten the health of humans. Therefore, a rapid and reliable sensor for OP detection is required. Amperometric acetylcholinesterase (AChE) biosensors have been shown as a suitable tool for OP detection due to their fast response, simplicity, convenience, and low-cost analysis. The crucial aspects to improve the performance of AChE biosensors are enhancement of conductivity and biocompatibility of modified electrode materials. Ma et al. developed an AChE biosensor with Pt nanoparticle-anchored zirconium-based metal–organic framework nanocomposites for the detection of OPs in the environment (Ma et al. 2019). Earlier, Pohanka et al. used an electrochemical sensor to provide information about cholinesterase activity in blood of rats intoxicated with paraoxon. Paraoxon, an organophosphate inhibiting AChE and butyrylcholinesterase (BuChE), is the active metabolite of the insecticide parathion. The blood cholinseterase activity represents an important marker of organophosphate intoxication. The authors declared that the electrochemical-based sensor provided a precise evaluation of cholinesterase activity (Pohanka et al. 2009).
Besides artificial pesticides, various chemicals are present in food that are important to detect including toxins, heavy metals, additives, antibiotics, and organic and inorganic contaminants. These compounds also decrease food quality and represent a potential risk for consumers. However, an advance in biosensor food monitoring is a promising approach to improve the quality of food. Kaur et al. reported a simple colorimetric and potentiometric biosensor construction based on urease inhibition by Pb2+ ions in milk samples. The lower detection limit for lead in colorimetric approach was 38.6 μM in water and milk samples. The lower limit of detection in the potentiometric approach was 9.66 μM, and the sensor detected leads specifically in the milk without any pretreatment (Kaur et al. 2014). Ramachandra et al. in 2016 reported that a biosensor for the detection of CaC2, an artificial ripening agent in mangoes that causes serious health issues (neurological disorders, ulcers, hypoxia, memory loss, etc.), has been developed. The biosensor comprises of CeO2-modified Pt electrode for the determination of CaC2 based on AChE enzyme inhibition. The developed biosensor showed good conductivity, biocompatibility, and enhanced electron transfer rate with the limit of detection of 0.6 nM and linear range of 1–20 nM and response time less than 4 s. The authors also suggested its potential application for the detection of CaC2 in other artificially ripened fruits (Ramachandra et al. 2016). Formaldehyde is considered a toxic compound since it has been classified as group 1 carcinogen to human. Despite this, it has been proved the use of formaldehyde in fish preservation, particularly in Asian countries. Ease, fast, and low-cost formaldehyde detection can be useful to protect consumers from poisoning. Therefore, Aini et al. turn their attention to the development of formaldehyde biosensor for the determination of formalin in fish samples. The biosensor is based on gold nanoparticles, ionic liquid, chitosan (nanocomposite membrane), and glassy carbon electrode. As a biorecognition receptor in the system, an enzyme formaldehyde dehydrogenase was used to ensure selectivity towards the substrate, formaldehyde. Under optimal conditions, a wider linear range of formaldehyde concentrations from 0.01 to 10 ppm within 5 s was detected using the differential pulse voltammetry, with the limit of detection of 0.1 ppm. The biosensor was successfully applied for the detection of formaldehyde presence in fish samples, Lutjanus malabaricus and Thunnus Tonggol. The developed method is highly accurate, rapid, and simple, compared to the existing technique (Noor Aini et al. 2016). Dervisevic et al. utilized an electrochemical biosensor based on a hybrid bionanocomposite platform in the evaluation of meat freshness assay. The authors in the study focused on the detection of xanthine, a product of adenosine-3-phosphate degradation, using a xanthine biosensor based on chitosan-polypyrrole-gold nanoparticles. Electrochemical studies were performed by the modified electrode with immobilized xanthine oxidase. The biosensor was tested on real samples of fish, beef, and chicken. Xanthine biosensor overall exhibited a very good linear range of 1–200 μM and low limit of detection of 0.25 μM with an average response time of 8 s. Study further revealed that the xanthine biosensor was not prone to significant interference from ascorbic acid, uric acid, glucose, and sodium benzoate (Dervisevic et al. 2017). An inadequate stocking food may also represent a potential risk for consumers. A wet and humid climate can be profitable for different types of moulds such as Aspergillus flavus or Aspergillus parasiticus producing aflatoxins. Aflatoxins represent secondary metabolites, products of a polyketide pathway. These toxins are considered as one of the most carcinogenic natural compounds causing hepatocellular carcinoma. Pohanka et al. developed a method for the detection of aflatoxin B1 in capsicum spice. The assay utilized an electrochemical immunosensor with immobilized aflatoxin B1-bovine serum albumin conjugate. The authors declared that the whole device and assay are very practical for application in real conditions (Pohanka et al. 2008).
Biosensors bear the potential to detect various diseases caused by microorganisms as well as to provide data about microbial viability. The development of ATP bioluminescence biosensors to determine bacterial viability in milk and other animal food have been reported (Eed et al. 2015). In 2012, Gramberg et al. reported the construction of a cell-based biosensor for the detection of plant viruses. The principle of detection was based on the measurement of bacterial membrane potential changes as a result of virus-antibody binding. Biosensor membranes have been engineered using modified Escherichia coli bacteria. The biosensor was applied for the detection of cherry leaf roll virus (CLRL) and tobacco mosaic virus (TMV). Interestingly, the virus detection limit of the biosensor was 1 pg/mL with assay time 60–100 s (Gramberg et al. 2012). Recently, a whole-cell-based biosensor to detect the presence of Penicillium digitatum infection in citrus fruit was developed and evaluated. Detection was based on the luminescent responses of bacteria to changes in volatile organic compounds following a pathogenic microorganism-mediated infection. Biosensors allowed to detect pathogen on the third day of infection, before the appearance of visible signs of fungal infection on the surface of the fruit (Chalupowicz et al. 2020). Izadi et al. described the fabrication of a DNA-based pencil graphite electrode modified by Au nanoparticles for the detection of Bacillus cereus in milk. Toxins produced by the bacteria cause two types of gastrointestinal illness, the emetic syndrome and diarrheal syndrome. The authors emphasized a rapid detection, low-cost, and high selectivity of the biosensor specially developed to detect target DNA sequence in the bacteria. Further benefits involve high reproducibility and stability of the biosensor (Izadi et al. 2016). Table 1 summarizes recent advances of biosensors in the agriculture and food industry.
Biosensors in pharmaceutical sciences
Biosensors allow us to study numerous compounds of the pharmaceutical interest and to provide real-time analysis of various processes. These helpful detectors may be employed at home (physiological condition control), in hospital (emergency, surgery, dialysis monitoring), in clinical laboratory analyses (DNA analysis, immunoassays), in laboratory research, and others. Ideal biosensor should be minimal size, low-cost, and easy to use, allowing direct and fast analysis without sample pretreatment. Measurement should be non-invasive, automatized, or remote-controlled (Kauffmann 2002).
Pathogens, such as algae, fungi, bacteria, viruses, and other parasites, are causative agents of various diseases; therefore, their rapid detection is crucial for a successful treatment. Detection of pathogens via biosensors brings a modern solution to an old problem. Various biosensors for pathogen detection have been developed by using electrical, electrochemical, mechanical, NMR, and optical sensing methods. Among the developed biosensors, optical, especially colorimetric sensors allow rapid, easy-to-use, portable, and cost-effective analysis (Yoo and Lee 2016). However, other biosensor types are also remarkable and advantageous for employment in health protection. Weerathunge et al. developed an ultrasensitive colorimetric detection of murine norovirus (MNV) using NanoZyme aptasensor. The strategy combines the gold nanoparticles enzyme-mimic catalytic activity with high target specificity of MNV aptamer. The present method achieved the most sensitive detection of the virus using a biosensor approach. The authors further demonstrated the robustness of the MNV NanoZyme aptasensor by its application in the presence of other non-target microorganisms. Developed ultrasensitive MNV detection is simple to use and rapid and eliminates the need for expensive instrumentation (Weerathunge et al. 2019). Pang et al. designed a fluorescent aptasensor system for the sensitive detection of recombinant hemagglutinin (rHA) protein of the influenza A subtype H5N1. Guanine-richen anti-rHA aptamers were immobilized on the surface of Ag-SiO2 nanoparticles which acted as a metal-enhanced fluorescent sensing platform. Thiazole orange was used as a fluorescent tag, which was free with almost no fluorescence emission in the solution without rHA. However, in solutions with rHA, the aptamer strand bound rHA protein to form a G-quadruplex complex that can bind thiazole orange and excite the fluorescence emission. The rHA protein of the influenza A subtype H5N1 was detected with the limit of detection of 2 ng/mL in aqueous buffer and 3.5 ng/mL in human serum. Moreover, the whole experiment process can be finished within 30 min (Pang et al. 2015). The human adenovirus diagnostic system was developed by Jin et al. in 2018. The methodology involves a novel DNA extraction technique based on the integration of a non-chaotropic reagent with a disposable thin-film platform in a single microchannel. To detect extracted viral DNA, isothermal solid-phase DNA amplification/detection optical biosensor was used. The authors validated the clinical utility of the system in human samples and concluded that this system enables a rapid (less than 1 h) and sensitive (100 times higher than qPCR) diagnostic platform for viral DNA analysis (Jin et al. 2018). A selective and sensitive electrochemical immunosensor for Zika virus (ZIKV) protein detection was elaborated by Kaushik et al. in 2018. In this approach, the authors utilized a functionalized integrated micro-electrode of gold (IDE-Au) array. Electrochemical immunosensor was fabricated via immobilization of ZIKV envelope protein antibody onto a self-assembled monolayer of dithiobis (succinimidyl propionate) deposited on IDE-Au. The developed biosensor allowed rapid analysis (operation time around 40 min) with a low detection limit of 10 pM and promising clinical application for early-stage diagnostics of the virus (Kaushik et al. 2018). Babamiri et al. fabricated a novel molecularly imprinted polymer electrochemiluminescence sensor for selective detection of HIV-1 gene with high sensitivity. As a signal producing compound, europium sulphide nanocrystals were used. The HIV aptamer as the template and o-phenylenediamine as the functional monomer were directly electropolymerized on the surface of the indium tin oxide electrode. The hybridization reaction was mediated between europium sulphide nanocrystal functionalized 5-amino-labelled oligonucleotides as capture probes and detection target (HIV gene) oligonucleotides. The electrochemiluminescence signal significantly increased using K2S2O8 as a coreactant. Selective and sensitive detection of the HIV gene was achieved in a linear range of 3.0 fM to 0.3 nM with a limit of detection of 0.3 fM. The authors further reported that the detection of HIV in real human serum samples was obtained with satisfactory results (Babamiri et al. 2018). Verma et al. recently developed an ultrasensitive DNA biosensor for a rapid detection of the Loa22 gene of Leptospira interrogans, a Gram-negative bacterium causing leptospirosis. The biosensor uses a gold nanoparticle–carbon nanofiber composite-based screen-printed electrode. The authors claimed that the DNA-based biosensor provided a rapid detection of the pathogen with a higher selectivity and specificity (Verma et al. 2021).
Since early of 2020, a global attention has been focusing on detection of viruses, specially to the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing an ongoing pandemic of coronavirus disease 2019 (COVID-19). Despite hugeous endeavour to reduce the spread of the virus, COVID-19 still remains an international threat. One of the most important factors to overcome the pandemic is an early detection of the virus. Currently, the most effective method for detection of SARS-CoV-2 is reverse transcriptase polymerase chain reaction (RT-PCR). However, an exponential application of RT-PCR as a gold standard in diagnostic of SARS-CoV-2 involves some disadvantages. For example, the method is time-consuming and requires specialized laboratory equipment and qualified personnel. On the other hand, antigen test for detection of SARS-CoV-2 is fast and easy to handle but has an increased possibility of false results. Therefore, it is not surprising that a continuous demand for the development of novel SARS-CoV-2 diagnostic methods results in development of novel biosensors. Diagnostic of the virus via biosensors is focused on the detection of RNA, antigens, and antibodies (Pradhan et al. 2021; Zare et al. 2021). Oui et al. developed a dual-functional biosensor based on the combination of plasmonic photothermal (PPT) effect and localized surface plasmon resonance (LSPR) sensing transduction. Sensitive detection of SARS-CoV-2 was mediated by using of two-dimensional gold nanoislands (AuNIs) functionalized with complementary DNA receptors. The biosensor allows precise detection of desired target in a multigene mixture with LOD of 0.22 pM for the RNA-dependent RNA polymerase (RdRp) (Qiu et al. 2020). An electrochemiluminescence (ECL) biosensor for a detection of RdRp gene of SARS-CoV-2 was developed by Fan et al. The biosensor utilizes DNA tetrahedron modified on the surface of electrode providing programmable scaffolds upon which target DNA participates. This results in entropy-driven amplified reaction proceeding through Ru(bpy)32+ modified S3 linked to the linear single-stranded DNA (ssDNA) at the vertex of the tetrahedron. The biosensor demonstrated considerable specificity and high selectivity towards the detection of the virus with LOD of 2.67 fM. The detection of RdRp of SARS-CoV-2 was achieved in human serum samples supporting its potential application in the clinical bioanalysis (Fan et al. 2021). Moitra et al. reported a selective and visual “naked-eye” detection of SARS-CoV-2 without any sophisticated instrumental techniques. The colorimetric assay is based on gold nanoparticles capped with thiol-modified antisense oligonucleotides specific for the N-gene of SARS-CoV-2. The gold nanoparticles capped with thiol-modified antisense oligonucleotides selectively agglomerate in the presence of the specific RNA sequence of SARS-CoV-2, resulting in a change of its SPR. Moreover, an addition of ribonuclease H yields in a cleavage of RNA strand from the RNA–DNA hybrid, which can be visually detected as a precipitate formed by the additional agglomeration between gold nanoparticles. Diagnostic of the virus can be achieved within 10 min of the isolated RNA samples. The authors selectively detected RNA from SARS-CoV-2 in the presence of RNA from the Middle East respiratory syndrome coronavirus (MERS-CoV) with LOD of 0.18 ng/μL (Moitra et al. 2020). Yakoh et al. developed a paper-based electrochemical biosensor to diagnose COVID-19. The methodology utilizes a sensitive and specific immunosensor detecting immunoglobulin produced against SARS-CoV-2. Sensing is based on the disruption of the redox conversion ([Fe(CN)6]3−/4−) caused by the presence of SARS-CoV-2 antibodies. The assay can be performed within 30 min with LOD of 1 ng/mL. It was reported that the electrochemical biosensor provided satisfactory results in real clinical sera. Moreover, the methodology for diagnosing COVID-19 can be extended to the detection of antigen (the spike protein of SARS-CoV-2) (Yakoh et al. 2021). Cady et al. reported a detection of antibodies against SARS-CoV-2 using a grating-coupled fluorescent plasmonic (GC-FP) biosensor platform in human blood serum and dried blood spot samples. The GC-FP platform is based on the measurement of antibody–antigen binding interactions. The methodology can be utilized to measure the levels of antibody for multiple antigens in a single sample. The assay is rapid (30 min) and quantitative across a large dynamic range, using serum dilutions in the range 1:25 to 1:1600. The authors concluded that deeper insight into antibody responses across different immunoglobulin classes could be useful for the viral analysis of other bodily fluids (Cady et al. 2021). Seo et al. developed field-effect transistor (FET)-based biosensor for the diagnosis of COVID-19. The FET biosensor is functionalized with a specific antibody against SARS-CoV-2 spike protein conjugated to graphene sheet. Moreover, the biosensor provided no cross-reactivity with MERS-CoV antigen. The determination of the virus in clinical samples was achieved without any pre-processing with a large dynamic range. The biosensor could detect spike protein of SARS-CoV-2 in phosphate-buffered saline at concentration of 1 fg/mL and in clinical transport medium at concentration of 100 fg/mL. In addition, the methodology has a promising potential to detect other emerging viral diseases (Seo et al. 2020). Chen et al. reported the development of lateral flow immunoassay (LFIA) using lanthanide-doped polystyrene nanoparticles (LNPs) for the detection of immunoglobulin G (IgG) antibody against SARS-CoV-2 in human serum. A nitrocellulose membrane functionalized with a recombinant nucleocapsid phosphoprotein of SARS-CoV-2 was utilized to capture the IgG. The methodology provided a simple and rapid detection of the virus. The assay can be also used to monitor the progression of COVID-19 in patient or to evaluate patient’s response to treatment (Chen et al. 2020).
Cancer is the second leading cause of death globally, responsible for about 10 million deaths per year. Most of the deaths from cancer (approximately 70%) occur in low- and middle-income countries (Sung et al. 2021). Early diagnosis of cancer significantly improves the chance for successful treatment. Therefore, several research groups focused their attention on the development of cancer diagnostics biosensors. Pacheco et al. fabricated a gold screen-printed electrode that was modified with a molecularly imprinted polymer to detect the breast cancer biomarker CA 15–3. The biosensors provided a short time analysis (15 min) with a detection limit of 1.5 U/mL that is well below the used cut-off value in clinical practice (25 U/mL). In addition, the simplicity and low-cost of the analysis contribute to the clinical application of the biosensor as a promising tool for the detection of breast cancer and to evaluate the progression/recurrence of cancer (Pacheco et al. 2018). Salahandish et al. developed a biosensor for the detection of HER2+ breast cancer with a significant signal enhancement by graphene nanostructured polyaniline and silver nanoparticles. In this sandwich-like model of functionalized nitrogen-doped multilayer structure, with a correct arrangement and combination of nanocomposites, excellent conductivity and stability were observed. This approach eliminated the need for any biological enzymes, making the system universal and simple. The biosensor with a dynamic range of 10 to 5 × 106 cells/mL achieved the detection limit of 2 cells/mL (Salahandish et al. 2018).
In recent years, the global popularity of smartphones resulted in the development of a new category of daily-using detectors, termed wearable biosensors. The popularity of wearable biosensors has a growing tendency due to their potential to provide real-time, continuous physiological analysis via measurements of biochemical markers in biofluids, such as sweat, saliva, tears, and interstitial fluid. Present developments are focusing on electrochemical and optical biosensors. Customers prefer multiplexed minimal size biosensors combined with flexible materials to improve wearability and ease of operation. To underpin a future clinical acceptance of wearable biosensors, further studies and development are needed; however, their broad impact on daily life is apparent already to date (Kim et al. 2019). A summary of recent advances and applications of biosensors in pharmaceutical sciences is presented in Table 2.
Biosensors in environmental sciences
One of the most promising aspects for the utilization of biosensors in the environment is the detection of pollutants in soil, water, and air. Monitoring in the environment is focused on the determination of heavy metals such as mercury, copper, cadmium, lead, zinc, and arsenic; nitrogen compounds, especially nitrates in the soil, groundwater, and surface water that can harm the health of human and aquatic life; phenolic compounds utilized in industry and manufactures; biochemical oxygen demand, a commonly used parameter for determination of environmental pollution; microorganisms; and other (Singh and Khajuria 2020). Benzene and its derivatives belong to compounds whose exposure poses a significant risk to human health. Ray et al. developed three high selective protein-based biosensors for the detection of benzene and its derivatives with the limit of detection of 0.3 ppm. Moreover, the developed biosensors were able to differentiate between methyl-substituted benzene derivatives (toluene, xylene and mesitylene) (Ray et al. 2018). An enzyme-based fibre optic biosensor for detection of halogenated hydrocarbon pollutants was fabricated by Shahar et al. in 2018. The approach is based on immobilized enzyme-mediated hydrolytic dehalogenation of halogenated aliphatic hydrocarbon to corresponding primary alcohol, halide ion, and proton. The proton concentration changes led to increased protonation of immobilized Nile blue chromoionophore, an optical pH indicator. The resulting optical change is monitored by a fibre optic reflectance spectrophotometer. The biosensor exhibited a dynamic linear response range of 1–30 mg/L with the limit of detection of 0.3 mg/L and rapid response (within 2 min), towards detection of 1,2-dichloroethane (Shahar et al. 2019). Conventional measuring methods of biochemical oxygen demand (BOD), a widely used index for determining the amount of biodegradable organic matter in wastewater, are time-consuming techniques (5 days); therefore, a more rapid method is required. Liang et al. constructed a flame-oxidized stainless steel anode as the probe of a bioelectrochemical system-based biosensor for the measurements of BOD. The evaluation was performed on real wastewater samples to simulate the practical application of the biosensor. As a result, the developed biosensor offered better performance than traditional carbon-cloth anodes in bioelectrochemical biosensors. The authors further proposed various practical utilizations of the biosensor to improve the biomonitoring of wastewater (Liang et al. 2018). The great challenge nowadays is the monitoring of the marine environment. Marine pollutants are hazardous to all forms of aquatic life, such as algae, plankton, crustaceans, fish, and marine mammals. An optical biosensor based on the array of algal biomediators has been developed by Turemis et al. for the measurements of pesticides in marine water. The algae–protozoa algae association between Chlorella vulgaris and Tetrahymena pyriformis was assessed as a sensitive biomediator for biosensor development. Entrapped symbiotic strain in calcium alginate within novel fluidic flow cells with integrated detectors provided real-time detection mediated by florescence analysis of photosynthetic photosystem II. The biosensor was examined to detect three commonly found pesticides (simazine, atrazine, and diuron), and results were compared with LC–MS analysis. Determined limits of detection were 1.35 μg/L for simazine, 0.44 μg/L for atrazine, 0.25 μg/L for diuron, and 0.13 μg/L for the mixture (Turemis et al. 2018). An optical biosensor for soil contamination assessment utilizing bioluminescent bacterial bioreporters encapsulated in poly-dopamine-coated alginate microbeads was fabricated by Bae et al. in 2020. In their approach, two bioluminescent reporter strains were employed for the detection of toluene as a model soil contaminant in the ambient light-blocking, temperature-controlled biosensor module. Bioluminescence of strain TV1061 (Escherichia coli) increased, whereas that of strain GC2 (Escherichia coli) decreased in the presence of increasing toluene concentrations. This simple optical biosensor can potentially be utilized for soil contamination monitoring in areas suspected of chemical pollution such as petrol stations or petrochemical industrial zone (Bae et al. 2020). Recent advances and applications of biosensors related to the environment are shown in Table 3.
Conclusions and future directions
Biosensors are a continuously developing field; various kinds of biosensors are available. Biosensors mainly include luminescence-based sensors, optical-based sensors, and carbon nanotube-based sensors. Recently developed sensors provide a high signal-to-noise ratio; they are cheap and can be quickly commercialized (Table 4). Biosensors have been developed for the detection of glucose tyrosine DNA and bacteria from various samples. The last few decades have seen a surge in biosensor research related to sensitivity enhancement and innovative target materials for specificity. Nano-technological advances have increased surface plasmon resonance-based biosensor research. Carbon-based nanomaterials, like graphene and its derivatives, have revolutionized the field of biosensing due to their extraordinary properties, such as large surface area, easy synthesis, tuneable optical properties, and strong compatible adsorption of biomolecules. Carbon nanotube-based materials have been used to act as a plasmonic layer to provide the large surface area and compatibility for immobilizing biomolecules, such as enzymes, DNA, antibodies, and antigens, in the design of the sensing layer. As a result, this field continues to be at the forefront of evolving biosensing technology. Carbon nanotube-based biosensors demonstrate an active growth of research interest in food analysis. The high specific surface area and catalytic activity of graphene are utilized for the extraction of analytes from food samples. Their high conductivity contributes to the performance efficacy of the electrodes. They have been utilized as transducers in biosensing platforms such as electrochemical, optical, chemo-resistive, and field-effect transistor-based biosensors for the detection of various analytes in food. We summarize the recent progress for the detection of chemical contaminants in food and the environment, together with clinical applications, and the principles of operation and discuss the perspectives of developing portable devices for rapid in-field analysis.
References
Afsahi S, Lerner MB, Goldstein JM, Lee J, Tang X, Bagarozzi DA, Pan D, Locascio L, Walker A, Barron F, Goldsmith BR (2018) Novel graphene-based biosensor for early detection of Zika virus infection. Biosens Bioelectron 100:85–88. https://doi.org/10.1016/j.bios.2017.08.051
Afsarimanesh N, Mukhopadhyay SC, Kruger M (2018) Molecularly imprinted polymer-based electrochemical biosensor for bone loss detection. IEEE Trans Biomed Eng 65:1264–1271. https://doi.org/10.1109/TBME.2017.2744667
Afsarimanesh N, Mukhopadhyay SC, Kruger M (2019) State-of-the-art of sensing technologies for monitoring of bone-health. In: Afsarimanesh N, Mukhopadhyay SC, Kruger M (eds) Electrochemical biosensor: point-of-care for early detection of bone loss. Springer International Publishing, Cham, pp 7–31
Alhadrami HA, Paton GI (2013) The potential applications of SOS-lux biosensors for rapid screening of mutagenic chemicals. FEMS MicrobiolLett 344:69–76. https://doi.org/10.1111/1574-6968.12156
Alleyne CJ, Kirk AG, McPhedran RC, Nicorovici N-AP, Maystre D (2007) Enhanced SPR sensitivity using periodic metallic structures. Opt Express 15:8163–8169. https://doi.org/10.1364/oe.15.008163
Andryukov BG, Besednova NN, Romashko RV, Zaporozhets TS, Efimov TA (2020) Label-free biosensors for laboratory-based diagnostics of infections: current achievements and new trends. Biosensors (Basel) 10. https://doi.org/10.3390/bios10020011
Angelopoulou Μ, Botsialas A, Salapatas A, Petrou PS, Haasnoot W, Makarona E, Jobst G, Goustouridis D, Siafaka-Kapadai A, Raptis I, Misiakos K, Kakabakos SE (2015) Assessment of goat milk adulteration with a label-free monolithically integrated optoelectronic biosensor. Anal Bioanal Chem 407:3995–4004. https://doi.org/10.1007/s00216-015-8596-3
Anik Ü, Tepeli Y, Diouani MF (2016) Fabrication of electrochemical model influenza A virus biosensor based on the measurements of neuroaminidase enzyme activity. Anal Chem 88:6151–6153. https://doi.org/10.1021/acs.analchem.6b01720
Arduini F, Cinti S, Scognamiglio V, Moscone D (2016) Nanomaterials in electrochemical biosensors for pesticide detection: advances and challenges in food analysis. Microchim Acta 183:2063–2083. https://doi.org/10.1007/s00604-016-1858-8
Ashiba H, Sugiyama Y, Wang X, Shirato H, Higo-Moriguchi K, Taniguchi K, Ohki Y, Fujimaki M (2017) Detection of norovirus virus-like particles using a surface plasmon resonance-assisted fluoroimmunosensor optimized for quantum dot fluorescent labels. Biosens Bioelectron 93:260–266. https://doi.org/10.1016/j.bios.2016.08.099
Babamiri B, Salimi A, Hallaj R (2018) A molecularly imprinted electrochemiluminescence sensor for ultrasensitive HIV-1 gene detection using EuS nanocrystals as luminophore. Biosens Bioelectron 117:332–339. https://doi.org/10.1016/j.bios.2018.06.003
Bachilo SM, Strano MS, Kittrell C, Hauge RH, Smalley RE, Weisman RB (2002) Structure-assigned optical spectra of single-walled carbon nanotubes. Science 298:2361–2366. https://doi.org/10.1126/science.1078727
Bae JW, Seo HB, Belkin S, Gu MB (2020) An optical detection module-based biosensor using fortified bacterial beads for soil toxicity assessment. Anal Bioanal Chem 412:3373–3381. https://doi.org/10.1007/s00216-020-02469-z
Bahadır EB, Sezgintürk MK (2015) Electrochemical biosensors for hormone analyses. Biosens Bioelectron 68:62–71. https://doi.org/10.1016/j.bios.2014.12.054
Bai H, Wang R, Hargis B, Lu H, Li Y (2012) A SPR aptasensor for detection of avian influenza virus H5N1. Sensors (basel) 12:12506–12518. https://doi.org/10.3390/s120912506
Balaji A, Zhang J (2017) Electrochemical and optical biosensors for early-stage cancer diagnosis by using graphene and graphene oxide. Cancer Nanotechnol 8. https://doi.org/10.1186/s12645-017-0035-z
Bhatta D, Villalba MM, Johnson CL, Emmerson GD, Ferris NP, King DP, Lowe CR (2012) Rapid detection of foot-and-mouth disease virus with optical microchip sensors. Procedia Chemistry 6:2–10. https://doi.org/10.1016/j.proche.2012.10.124
Bidmanova S, Kotlanova M, Rataj T, Damborsky J, Trtilek M, Prokop Z (2016) Fluorescence-based biosensor for monitoring of environmental pollutants: from concept to field application. Biosens Bioelectron 84:97–105. https://doi.org/10.1016/j.bios.2015.12.010
Bobrinetskiy II, Knezevic NZ (2018) Graphene-based biosensors for on-site detection of contaminants in food. Anal Methods 10:5061–5070. https://doi.org/10.1039/C8AY01913D
Bolton EK, Sayler GS, Nivens DE, Rochelle JM, Ripp S, Simpson ML (2002) Integrated CMOS photodetectors and signal processing for very low-level chemical sensing with the bioluminescent bioreporter integrated circuit. Sens Actuators, B Chem 85:179–185. https://doi.org/10.1016/S0925-4005(02)00106-5
Borghei G, Hall EAH (2014) BRET-linked ATP assay with luciferase. Analyst 139:4185–4192. https://doi.org/10.1039/C4AN00436A
Brar SK, Hegde K, Pachapur VL (2019) Tools, techniques and protocols for monitoring environmental contaminants. Elsevier
Brogioni B, Berti F (2014) Surface plasmon resonance for the characterization of bacterial polysaccharide antigens: a review. Med Chem Commun 5:1058–1066. https://doi.org/10.1039/C4MD00088A
Cady NC, Tokranova N, Minor A, Nikvand N, Strle K, Lee WT, Page W, Guignon E, Pilar A, Gibson GN (2021) Multiplexed detection and quantification of human antibody response to COVID-19 infection using a plasmon enhanced biosensor platform. Biosens Bioelectron 171:112679. https://doi.org/10.1016/j.bios.2020.112679
Cai H, Parks JW, Wall TA, Stott MA, Stambaugh A, Alfson K, Griffiths A, Mathies RA, Carrion R, Patterson JL, Hawkins AR, Schmidt H (2015) Optofluidic analysis system for amplification-free, direct detection of Ebola infection. Sci Rep 5:14494. https://doi.org/10.1038/srep14494
Campuzano S, Ruiz-Valdepeñas Montiel V, Serafín V, Yáñez-Sedeño P, Pingarrón JM (2020) Cutting-edge advances in electrochemical affinity biosensing at different molecular level of emerging food allergens and adulterants. Biosensors (Basel) 10. https://doi.org/10.3390/bios10020010
Cao J, Sun T, Grattan KTV (2014) Gold nanorod-based localized surface plasmon resonance biosensors: a review. Sens Actuators, B Chem 195:332–351. https://doi.org/10.1016/j.snb.2014.01.056
Caputo D, de Cesare G, Dolci LS, Mirasoli M, Nascetti A, Roda A, Scipinotti R (2013) Microfluidic chip with integrated a-Si: H photodiodes for chemiluminescence-based bioassays. IEEE Sens J 13:2595–2602. https://doi.org/10.1109/JSEN.2013.2256889
Cesewski E, Johnson BN (2020) Electrochemical biosensors for pathogen detection. Biosens Bioelectron 159:112214. https://doi.org/10.1016/j.bios.2020.112214
Chalupowicz D, Veltman B, Droby S, Eltzov E (2020) Evaluating the use of biosensors for monitoring of Penicillium digitatum infection in citrus fruit. Sens Actuators B Chem 311:127896. https://doi.org/10.1016/j.snb.2020.127896
Chappelle EW, Levin GV (1968) Use of the firefly bioluminescent reaction for rapid detection and counting of bacteria. Biochem Med 2:41–52. https://doi.org/10.1016/0006-2944(68)90006-9
Chauhan N, Narang J, Jain U (2016) Amperometric acetylcholinesterase biosensor for pesticides monitoring utilising iron oxide nanoparticles and poly(indole-5-carboxylic acid). J Exp Nanosci 11:111–122. https://doi.org/10.1080/17458080.2015.1030712
Cheeveewattanagul N, Morales-Narváez E, Hassan A-RHA, Bergua JF, Surareungchai W, Somasundrum M, Merkoçi A (2017) Straightforward immunosensing platform based on graphene oxide-decorated nanopaper: a highly sensitive and fast biosensing approach. Adv Func Mater 27:1702741. https://doi.org/10.1002/adfm.201702741
Chen Z, Berciaud S, Nuckolls C, Heinz TF, Brus LE (2010) Energy transfer from individual semiconductor nanocrystals to graphene. ACS Nano 4:2964–2968. https://doi.org/10.1021/nn1005107
Chen Z, Zhang Z, Zhai X, Li Y, Lin L, Zhao H, Bian L, Li P, Yu L, Wu Y, Lin G (2020) Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal Chem 92:7226–7231. https://doi.org/10.1021/acs.analchem.0c00784
Cheng N, Du D, Wang X, Liu D, Xu W, Luo Y, Lin Y (2019) Recent advances in biosensors for detecting cancer-derived exosomes. Trends Biotechnol 37:1236–1254. https://doi.org/10.1016/j.tibtech.2019.04.008
Chong JPC, Liew OW, Li BQ, Asundi AK (2007) Optical fluorescence biosensor for plant water stress detection. 6535:65350S.https://doi.org/10.1117/12.740959
Chung H, Li J, Kim Y, Van Os JMC, Brounts SH, Choi CY (2020) Using implantable biosensors and wearable scanners to monitor dairy cattle’s core body temperature in real-time. Comput Electron Agric 174:105453. https://doi.org/10.1016/j.compag.2020.105453
Créton R, Jaffe LF (2001) Chemiluminescence microscopy as a tool in biomedical research. Biotechniques 31(1098–1100):1102–1105. https://doi.org/10.2144/01315rv01
Cunha I, Biltes R, Sales M, Vasconcelos V (2018) Aptamer-based biosensors to detect aquatic phycotoxins and cyanotoxins. Sensors (Basel) 18. https://doi.org/10.3390/s18072367
De Michele R, Carimi F, Frommer WB (2014) Mitochondrial biosensors. Int J Biochem Cell Biol 48:39–44. https://doi.org/10.1016/j.biocel.2013.12.014
Deng J, Toh C-S (2013) Impedimetric DNA biosensor based on a nanoporous alumina membrane for the detection of the specific oligonucleotide sequence of Dengue virus. Sensors 13:7774–7785. https://doi.org/10.3390/s130607774
Dervisevic M, Dervisevic E, Çevik E, Şenel M (2017) Novel electrochemical xanthine biosensor based on chitosan–polypyrrole–gold nanoparticles hybrid bio-nanocomposite platform. J Food Drug Anal 25:510–519. https://doi.org/10.1016/j.jfda.2016.12.005
Di Fusco M, Quintavalla A, Lombardo M, Guardigli M, Mirasoli M, Trombini C, Roda A (2015) Organically modified silica nanoparticles doped with new acridine-1,2-dioxetane analogues as thermochemiluminescence reagentless labels for ultrasensitive immunoassays. Anal Bioanal Chem 407:1567–1576. https://doi.org/10.1007/s00216-014-8406-3
Dominguez RB, Hayat A, Alonso GA, Gutiérrez JM, Muñoz R, Marty J-L (2017) 19 - Nanomaterial-based biosensors for food contaminant assessment. In: Grumezescu AM (ed) Nanobiosensors. Academic Press, pp 805–839
Dudak FC, Boyaci İH (2014) Peptide-based surface plasmon resonance biosensor for detection of staphylococcal enterotoxin B. Food Anal Methods 7:506–511. https://doi.org/10.1007/s12161-013-9739-9
Dudchenko OY, Pyeshkova VM, Soldatkin OO, Akata B, Kasap BO, Soldatkin AP, Dzyadevych SV (2016) Development of silicalite/glucose oxidase-based biosensor and its application for glucose determination in juices and nectars. Nanoscale Res Lett 11.https://doi.org/10.1186/s11671-016-1275-2
Eed HR, Abdel-Kader NS, El Tahan MH, Dai T, Amin R (2015) Bioluminescence-sensing assay for microbial growth recognition. Journal of Sensors 2016:e1492467. https://doi.org/10.1155/2016/1492467
El-Kady MF, Kaner RB (2013) Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat Commun 4:1475. https://doi.org/10.1038/ncomms2446
Ensafi AA, Karbalaei S, Heydari-Bafrooei E, Rezaei B (2016) Biosensing of naringin in marketed fruits and juices based on its interaction with DNA. J Iran Chem Soc 13:19–27. https://doi.org/10.1007/s13738-015-0707-8
Erdem Ö, Saylan Y, Cihangir N, Denizli A (2019) Molecularly imprinted nanoparticles based plasmonic sensors for real-time Enterococcus faecalis detection. Biosens Bioelectron 126:608–614. https://doi.org/10.1016/j.bios.2018.11.030
Erden PE, Kılıç E (2013) A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 107:312–323. https://doi.org/10.1016/j.talanta.2013.01.043
Ettenauer J, Zuser K, Kellner K, Posnicek T, Brandl M (2015) Development of an automated biosensor for rapid detection and quantification of E. coli in water. Procedia Engineering 120:376–379. https://doi.org/10.1016/j.proeng.2015.08.643
Evtugyn G, Subjakova V, Melikishvili S, Hianik T (2018) Affinity biosensors for detection of mycotoxins in food. Adv Food Nutr Res 85:263–310. https://doi.org/10.1016/bs.afnr.2018.03.003
Fan X, White IM, Shopova SI, Zhu H, Suter JD, Sun Y (2008) Sensitive optical biosensors for unlabeled targets: a review. Anal Chim Acta 620:8–26. https://doi.org/10.1016/j.aca.2008.05.022
Fan Z, Yao B, Ding Y, Zhao J, Xie M, Zhang K (2021) Entropy-driven amplified electrochemiluminescence biosensor for RdRp gene of SARS-CoV-2 detection with self-assembled DNA tetrahedron scaffolds. Biosens Bioelectron 178:113015. https://doi.org/10.1016/j.bios.2021.113015
Fatoni A, Numnuam A, Kanatharana P, Limbut W, Thammakhet C, Thavarungkul P (2013) A highly stable oxygen-independent glucose biosensor based on a chitosan-albumin cryogel incorporated with carbon nanotubes and ferrocene. Sens Actuators B Chem 185:725–734. https://doi.org/10.1016/j.snb.2013.05.056
Fukuda M, Wakuta S, Kamiyo J, Fujiwara T, Takano J (2018) Establishment of genetically encoded biosensors for cytosolic boric acid in plant cells. Plant J 95:763–774. https://doi.org/10.1111/tpj.13985
Gahlaut A, Hooda V, Gothwal A, Hooda V (2019) Enzyme-based ultrasensitive electrochemical biosensors for rapid assessment of nitrite toxicity: recent advances and perspectives. Crit Rev Anal Chem 49:32–43. https://doi.org/10.1080/10408347.2018.1461551
Gao G, Qian J, Fang D, Yu Y, Zhi J (2016) Development of a mediated whole cell-based electrochemical biosensor for joint toxicity assessment of multi-pollutants using a mixed microbial consortium. Anal Chim Acta 924:21–28. https://doi.org/10.1016/j.aca.2016.04.011
Gao G, Fang D, Yu Y, Wu L, Wang Y, Zhi J (2017) A double-mediator based whole cell electrochemical biosensor for acute biotoxicity assessment of wastewater. Talanta 167:208–216. https://doi.org/10.1016/j.talanta.2017.01.081
Garzón V, Pinacho DG, Bustos R-H, Garzón G, Bustamante S (2019) Optical biosensors for therapeutic drug monitoring. Biosensors 9:132. https://doi.org/10.3390/bios9040132
Gatel L, Cuprys A, Kumar P, Suresh G, Bendourou F, Chaali M, Hegde K, Brar SK (2019) Chapter 7 - Recent advances in oligonucleotide-based sensor technology for detection of endocrine-disrupting chemicals (EDC) in the environment. In: Kaur Brar S, Hegde K, Pachapur VL (eds) Tools, techniques and protocols for monitoring environmental contaminants. Elsevier, pp 147–167
Ge L, Liu Q, Hao N, Kun W (2019) Recent developments of photoelectrochemical biosensors for food analysis. J Mater Chem B 7:7283–7300. https://doi.org/10.1039/C9TB01644A
Gramberg B, Kintzios S, Schmidt U, Mewis I, Ulrichs C (2012) A basic approach towards the development of bioelectric bacterial biosensors for the detection of plant viruses. J Phytopathol 160:106–111. https://doi.org/10.1111/j.1439-0434.2011.01867.x
Grześkowiak BF, Tuśnio K, Woźniak A, Szalata M, Lipiński D, Jurga S, Słomski R (2019) Transgenic plant detection using an AuNPs based SPR biosensor. Biosensors (Basel) 9.https://doi.org/10.3390/bios9040116
Hassan SHA, Van Ginkel SW, Hussein MAM, Abskharon R, Oh S-E (2016) Toxicity assessment using different bioassays and microbial biosensors. Environ Int 92–93:106–118. https://doi.org/10.1016/j.envint.2016.03.003
Hassen WM, Chaix C, Abdelghani A, Bessueille F, Leonard D, Jaffrezic-Renault N (2008) An impedimetric DNA sensor based on functionalized magnetic nanoparticles for HIV and HBV detection. Sens Actuators, B Chem 134:755–760. https://doi.org/10.1016/j.snb.2008.06.020
Heineman WR, Karube I, Schmid RD, Turner APF (2016) Biosensors 92 Proceedings: The Second World Congress on Biosensors. Elsevier
Hosseinkhani S (2011) Molecular enigma of multicolor bioluminescence of firefly luciferase. Cell Mol Life Sci 68:1167–1182. https://doi.org/10.1007/s00018-010-0607-0
Huang X, Liang Y, Ruan L, Ren J (2014) Chemiluminescent detection of cell apoptosis enzyme by gold nanoparticle-based resonance energy transfer assay. Anal Bioanal Chem 406:5677–5684. https://doi.org/10.1007/s00216-013-7611-9
Huang Y, Shi M, Zhao L, Zhao S, Hu K, Chen Z-F, Chen J, Liang H (2014) Carbon nanotube signal amplification for ultrasensitive fluorescence polarization detection of DNA methyltransferase activity and inhibition. Biosens Bioelectron 54:285–291. https://doi.org/10.1016/j.bios.2013.10.065
Huang X, Liu Y, Yung B, Xiong Y, Chen X (2017) Nanotechnology-enhanced no-wash biosensors for in vitro diagnostics of cancer. ACS Nano 11:5238–5292. https://doi.org/10.1021/acsnano.7b02618
Hummelen JC, Luider TM, Wynberg H (1986) [39] Stable 1,2-dioxetanes as labels for thermochemiluminescent immunoassay. In: Methods in Enzymology. Academic Press, pp 531–557
Hummelen JC, Luider TM, Wynberg H (1988) Thermochemiluminescence immunoassay. EPRINTS-BOOK-TITLE
Ilkhani H, Farhad S (2018) A novel electrochemical DNA biosensor for Ebola virus detection. Anal Biochem 557:151–155. https://doi.org/10.1016/j.ab.2018.06.010
İstek MM, Erdem MM, Gürsan AE (2019) Impedimetric nanobiosensor for the detection of sequence-selective DNA hybridization. Hacettepe Journal of Biology and Chemistry 46:495–503
Izadi Z, Sheikh-Zeinoddin M, Ensafi AA, Soleimanian-Zad S (2016) Fabrication of an electrochemical DNA-based biosensor for Bacillus cereus detection in milk and infant formula. Biosens Bioelectron 80:582–589. https://doi.org/10.1016/j.bios.2016.02.032
Jahanshahi P, Zalnezhad E, Sekaran SD, Adikan FRM (2014) Rapid immunoglobulin M-based dengue diagnostic test using surface plasmon resonance biosensor. Sci Rep 4:3851. https://doi.org/10.1038/srep03851
Jin W, Maduraiveeran G (2017) Electrochemical detection of chemical pollutants based on gold nanomaterials. Trends in Environmental Analytical Chemistry 14:28–36. https://doi.org/10.1016/j.teac.2017.05.001
Jin CE, Lee TY, Koo B, Sung H, Kim S-H, Shin Y (2018) Rapid virus diagnostic system using bio-optical sensor and microfluidic sample processing. Sens Actuators B Chem 255:2399–2406. https://doi.org/10.1016/j.snb.2017.08.197
Jung S-H, Jang H, Lim M-C, Kim J-H, Shin K-S, Kim SM, Kim H-Y, Kim Y-R, Jeon T-J (2015) Chromatic biosensor for detection of phosphinothricin acetyltransferase by use of polydiacetylene vesicles encapsulated within automatically generated immunohydrogel beads. Anal Chem 87:2072–2078. https://doi.org/10.1021/ac501795x
Kabessa Y, Eyal O, Bar-On O, Korouma V, Yagur-Kroll S, Belkin S, Agranat AJ (2016) Standoff detection of explosives and buried landmines using fluorescent bacterial sensor cells. Biosens Bioelectron 79:784–788. https://doi.org/10.1016/j.bios.2016.01.011
Kauffmann JM (2002) Biosensors in the pharmaceutical domain. Ann Pharm Fr 60:28–37
Kaur H, Kumar S, Verma N (2014) Enzyme-based colorimetric and potentiometric biosensor for detecting Pb (II) ions in milk. Braz Arch Biol Technol 57:613–619. https://doi.org/10.1590/S1516-8913201402160
Kaushik A, Yndart A, Kumar S, Jayant RD, Vashist A, Brown AN, Li C-Z, Nair M (2018) A sensitive electrochemical immunosensor for label-free detection of Zika-virus protein. Sci Rep 8.https://doi.org/10.1038/s41598-018-28035-3
Khanmohammadi A, Aghaie A, Vahedi E, Qazvini A, Ghanei M, Afkhami A, Hajian A, Bagheri H (2020) Electrochemical biosensors for the detection of lung cancer biomarkers: a review. Talanta 206:120251. https://doi.org/10.1016/j.talanta.2019.120251
Kim J, Imani S, de Araujo WR, Warchall J, Valdés-Ramírez G, Paixão TRLC, Mercier PP, Wang J (2015) Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens Bioelectron 74:1061–1068. https://doi.org/10.1016/j.bios.2015.07.039
Kim J, Campbell AS, de Ávila BE-F, Wang J (2019) Wearable biosensors for healthcare monitoring. Nat Biotechnol 37:389–406. https://doi.org/10.1038/s41587-019-0045-y
Kriss R, Pieper KJ, Parks J, Edwards MA (2021) Challenges of detecting lead in drinking water using at-home test kits. Environ Sci Technol 55:1964–1972. https://doi.org/10.1021/acs.est.0c07614
Kundu M, Krishnan P, Kotnala RK, Sumana G (2019) Recent developments in biosensors to combat agricultural challenges and their future prospects. Trends Food Sci Technol 88:157–178. https://doi.org/10.1016/j.tifs.2019.03.024
Larrieu A, Champion A, Legrand J, Lavenus J, Mast D, Brunoud G, Oh J, Guyomarc’h S, Pizot M, Farmer EE, Turnbull C, Vernoux T, Bennett MJ, Laplaze L (2015) A fluorescent hormone biosensor reveals the dynamics of jasmonate signalling in plants. Nat Commun 6:6043. https://doi.org/10.1038/ncomms7043
Lee J, Morita M, Takemura K, Park EY (2018) A multi-functional gold/iron-oxide nanoparticle-CNT hybrid nanomaterial as virus DNA sensing platform. Biosens Bioelectron 102:425–431. https://doi.org/10.1016/j.bios.2017.11.052
Lengger S, Otto J, Elsässer D, Schneider O, Tiehm A, Fleischer J, Niessner R, Seidel M (2014) Oligonucleotide microarray chip for the quantification of MS2, ΦX174, and adenoviruses on the multiplex analysis platform MCR 3. Anal Bioanal Chem 406:3323–3334. https://doi.org/10.1007/s00216-014-7641-y
Li J, Chen L, Du L, Li M (2013) Cage the firefly luciferin! - a strategy for developing bioluminescent probes. Chem Soc Rev 42:662–676. https://doi.org/10.1039/c2cs35249d
Li N, Liu D, Cui H (2014) Metal-nanoparticle-involved chemiluminescence and its applications in bioassays. Anal Bioanal Chem 406:5561–5571. https://doi.org/10.1007/s00216-014-7901-x
Li X, Scida K, Crooks RM (2015) Detection of hepatitis B virus DNA with a paper electrochemical sensor. Anal Chem 87:9009–9015. https://doi.org/10.1021/acs.analchem.5b02210
Li N, Larin EM, Kerman K (2017) A miniaturized impedimetric immunosensor for the competitive detection of adrenocorticotropic hormone. Sensors 17:2836. https://doi.org/10.3390/s17122836
Liang Q, Yamashita T, Yamamoto-Ikemoto R, Yokoyama H (2018) Flame-oxidized stainless-steel anode as a probe in bioelectrochemical system-based biosensors to monitor the biochemical oxygen demand of wastewater. Sensors 18:607. https://doi.org/10.3390/s18020607
Liedberg B, Nylander C, Lunström I (1983) Surface plasmon resonance for gas detection and biosensing. Sensors and Actuators 4:299–304. https://doi.org/10.1016/0250-6874(83)85036-7
Liedberg B, Lundström I, Stenberg E (1993) Principles of biosensing with an extended coupling matrix and surface plasmon resonance. Sens Actuators, B Chem 11:63–72. https://doi.org/10.1016/0925-4005(93)85239-7
Lim JM, Kim JH, Ryu MY, Cho CH, Park TJ, Park JP (2018) An electrochemical peptide sensor for detection of dengue fever biomarker NS1. Anal Chim Acta 1026:109–116. https://doi.org/10.1016/j.aca.2018.04.005
Lin K-C, Lin Y-C, Chen S-M (2013) A highly sensitive nonenzymatic glucose sensor based on multi-walled carbon nanotubes decorated with nickel and copper nanoparticles. Electrochim Acta 96:164–172. https://doi.org/10.1016/j.electacta.2013.02.098
Liu Y, Shen T, Hu L, Gong H, Chen C, Chen X, Cai C (2017) Development of a thermosensitive molecularly imprinted polymer resonance light scattering sensor for rapid and highly selective detection of hepatitis A virus in vitro. Sens Actuators, B Chem 253:1188–1193. https://doi.org/10.1016/j.snb.2017.07.166
Lv M, Liu Y, Geng J, Kou X, Xin Z, Yang D (2018) Engineering nanomaterials-based biosensors for food safety detection. Biosens Bioelectron 106:122–128. https://doi.org/10.1016/j.bios.2018.01.049
Ma L, He Y, Wang Y, Wang Y, Li R, Huang Z, Jiang Y, Gao J (2019) Nanocomposites of Pt nanoparticles anchored on UiO66-NH2 as carriers to construct acetylcholinesterase biosensors for organophosphorus pesticide detection. Electrochim Acta 318:525–533. https://doi.org/10.1016/j.electacta.2019.06.110
Marquette CA, Blum LJ (2010) Chapter 14: Chemiluminescent and bioluminescent biosensors. In: Chemiluminescence and Bioluminescence. pp 488–510
Marrazza G (2014) Piezoelectric biosensors for organophosphate and carbamate pesticides: a review. Biosensors 4:301–317. https://doi.org/10.3390/bios4030301
Marzocchi E, Grilli S, Della Ciana L, Prodi L, Mirasoli M, Roda A (2008) Chemiluminescent detection systems of horseradish peroxidase employing nucleophilic acylation catalysts. Anal Biochem 377:189–194. https://doi.org/10.1016/j.ab.2008.03.020
Matsubara K, Kawata S, Minami S (1988) Optical chemical sensor based on surface plasmon measurement. Appl Opt 27:1160–1163. https://doi.org/10.1364/AO.27.001160
Mejri Omrani N, Hayat A, Korri-Youssoufi H, Marty JL (2016) Electrochemical biosensors for food security: mycotoxins detection. In: Nikolelis DP, Nikoleli G-P (eds) Biosensors for security and bioterrorism applications. Springer International Publishing, Cham, pp 469–490
Mello LD, Kisner A, Goulart MOF, Kubota LT (2013) Biosensors for antioxidant evaluation in biological systems. Comb Chem High Throughput Screen 16:109–120. https://doi.org/10.2174/1386207311316020005
Mittal S, Kaur H, Gautam N, Mantha AK (2017) Biosensors for breast cancer diagnosis: a review of bioreceptors, biotransducers and signal amplification strategies. Biosens Bioelectron 88:217–231. https://doi.org/10.1016/j.bios.2016.08.028
Mofford DM, Reddy GR, Miller SC (2014) Aminoluciferins extend firefly luciferase bioluminescence into the near-infrared and can be preferred substrates over d-luciferin. J Am Chem Soc 136:13277–13282. https://doi.org/10.1021/ja505795s
Moitra P, Alafeef M, Dighe K, Frieman MB, Pan D (2020) Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 14:7617–7627. https://doi.org/10.1021/acsnano.0c03822
Morales-Narváez E, Golmohammadi H, Naghdi T, Yousefi H, Kostiv U, Horák D, Pourreza N, Merkoçi A (2015) Nanopaper as an optical sensing platform. ACS Nano 9:7296–7305. https://doi.org/10.1021/acsnano.5b03097
Morales-Narváez E, Baptista-Pires L, Zamora-Gálvez A, Merkoçi A (2017) Graphene-based biosensors: going simple. Adv Mater 29:1604905. https://doi.org/10.1002/adma.201604905
Nguyen V-T, Kwon YS, Gu MB (2017) Aptamer-based environmental biosensors for small molecule contaminants. Curr Opin Biotechnol 45:15–23. https://doi.org/10.1016/j.copbio.2016.11.020
Niwa K, Ichino Y, Kumata S, Nakajima Y, Hiraishi Y, Kato D-I, Viviani VR, Ohmiya Y (2010) Quantum yields and kinetics of the firefly bioluminescence reaction of beetle luciferases. Photochem Photobiol 86:1046–1049. https://doi.org/10.1111/j.1751-1097.2010.00777.x
Nomngongo PN, Catherine Ngila J, Msagati TAM, Gumbi BP, Iwuoha EI (2012) Determination of selected persistent organic pollutants in wastewater from landfill leachates, using an amperometric biosensor. Physics and Chemistry of the Earth, Parts a/b/c 50–52:252–261. https://doi.org/10.1016/j.pce.2012.08.001
Noor Aini B, Siddiquee S, Ampon K (2016) Development of formaldehyde biosensor for determination of formalin in fish samples; malabar red snapper (Lutjanus malabaricus) and longtail tuna (Thunnus tonggol). Biosensors (Basel) 6. https://doi.org/10.3390/bios6030032
Oldach L, Zhang J (2014) Genetically encoded fluorescent biosensors for live-cell visualization of protein phosphorylation. Chem Biol 21:186–197. https://doi.org/10.1016/j.chembiol.2013.12.012
Osman AM, Zomer G, Laane C, Hilhorst R (2000) Comparative studies of the chemiluminescent horseradish peroxidase-catalysed peroxidation of acridan (GZ-11) and luminol reactions: effect of pH and scavengers of reactive oxygen species on the light intensity of these systems. Luminescence 15:189–197. https://doi.org/10.1002/1522-7243(200005/06)15:3%3c189::aid-bio585%3e3.0.co;2-a
Pacheco JG, Silva MSV, Freitas M, Nouws HPA, Delerue-Matos C (2018) Molecularly imprinted electrochemical sensor for the point-of-care detection of a breast cancer biomarker (CA 15–3). Sens Actuators, B Chem 256:905–912. https://doi.org/10.1016/j.snb.2017.10.027
Pang Y, Rong Z, Wang J, Xiao R, Wang S (2015) A fluorescent aptasensor for H5N1 influenza virus detection based-on the core-shell nanoparticles metal-enhanced fluorescence (MEF). Biosens Bioelectron 66:527–532. https://doi.org/10.1016/j.bios.2014.10.052
Pang Y, Jian J, Tu T, Yang Z, Ling J, Li Y, Wang X, Qiao Y, Tian H, Yang Y, Ren T-L (2018) Wearable humidity sensor based on porous graphene network for respiration monitoring. Biosens Bioelectron 116:123–129. https://doi.org/10.1016/j.bios.2018.05.038
Park JY, Kricka LJ (2014) Prospects for the commercialization of chemiluminescence-based point-of-care and on-site testing devices. Anal Bioanal Chem 406:5631–5637. https://doi.org/10.1007/s00216-014-7697-8
Park J-M, Jung H-W, Chang YW, Kim H-S, Kang M-J, Pyun J-C (2015) Chemiluminescence lateral flow immunoassay based on Pt nanoparticle with peroxidase activity. Anal Chim Acta 853:360–367. https://doi.org/10.1016/j.aca.2014.10.011
Peltomaa R, Glahn-Martínez B, Benito-Peña E, Moreno-Bondi MC (2018) Optical biosensors for label-free detection of small molecules. Sensors (Basel) 18. https://doi.org/10.3390/s18124126
Peng F, Su Y, Zhong Y, Fan C, Lee S-T, He Y (2014) Silicon nanomaterials platform for bioimaging, biosensing, and cancer therapy. Acc Chem Res 47:612–623. https://doi.org/10.1021/ar400221g
Pham HTM, Giersberg M, Gehrmann L, Hettwer K, Tuerk J, Uhlig S, Hanke G, Weisswange P, Simon K, Baronian K, Kunze G (2015) The determination of pharmaceuticals in wastewater using a recombinant Arxula adeninivorans whole cell biosensor. Sens Actuators B Chem 211:439–448. https://doi.org/10.1016/j.snb.2015.01.107
Piro B, Shi S, Reisberg S, Noël V, Anquetin G (2016) Comparison of electrochemical immunosensors and aptasensors for detection of small organic molecules in environment, food safety, clinical and public security. Biosensors (Basel) 6. https://doi.org/10.3390/bios6010007
Pohanka M, Malir F, Roubal T, Kuca K (2008) Detection of aflatoxins in capsicum spice using an electrochemical immunosensor. Anal Lett 41:2344–2353. https://doi.org/10.1080/00032710802350518
Pohanka M, Novotný L, Misík J, Kuca K, Zdarova-Karasova J, Hrabinova M (2009) Evaluation of cholinesterase activities during in vivo intoxication using an electrochemical sensor strip - correlation with intoxication symptoms. Sensors (basel) 9:3627–3634. https://doi.org/10.3390/s90503627
Pollap A, Kochana J (2019) Electrochemical immunosensors for antibiotic detection. Biosensors 9:61. https://doi.org/10.3390/bios9020061
Pourasl AH, Ahmadi MT, Rahmani M, Chin HC, Lim CS, Ismail R, Tan MLP (2014) Analytical modeling of glucose biosensors based on carbon nanotubes. Nanoscale Res Lett 9:33. https://doi.org/10.1186/1556-276X-9-33
Prabowo BA, Wang RYL, Secario MK, Ou P-T, Alom A, Liu J-J, Liu K-C (2017) Rapid detection and quantification of Enterovirus 71 by a portable surface plasmon resonance biosensor. Biosens Bioelectron 92:186–191. https://doi.org/10.1016/j.bios.2017.01.043
Pradhan A, Lahare P, Sinha P, Singh N, Gupta B, Kuca K, Ghosh KK, Krejcar O (2021) Biosensors as nano-analytical tools for COVID-19 detection. Sensors 21:7823. https://doi.org/10.3390/s21237823
Pundir CS, Chauhan N (2012) Acetylcholinesterase inhibition-based biosensors for pesticide determination: a review. Anal Biochem 429:19–31. https://doi.org/10.1016/j.ab.2012.06.025
Pyati R, Richter MM (2007) ECL—electrochemical luminescence. Annu Rep Prog Chem Sect c: Phys Chem 103:12–78. https://doi.org/10.1039/B605635K
Qiu G, Gai Z, Tao Y, Schmitt J, Kullak-Ublick GA, Wang J (2020) Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano 14:5268–5277. https://doi.org/10.1021/acsnano.0c02439
Ramachandra BL, Gumpu MB, Nesakumar N, Krishnan UM, Rayappan JBB (2016) Calcium carbide in mangoes: an electrochemical way for detection. Anal Methods 8:4590–4599. https://doi.org/10.1039/C6AY01314G
Ramanathan M, Patil M, Epur R, Yun Y, Shanov V, Schulz M, Heineman WR, Datta MK, Kumta PN (2016) Gold-coated carbon nanotube electrode arrays: immunosensors for impedimetric detection of bone biomarkers. Biosens Bioelectron 77:580–588. https://doi.org/10.1016/j.bios.2015.10.014
Randriamampita C, Lellouch AC (2014) Imaging early signaling events in T lymphocytes with fluorescent biosensors. Biotechnol J 9:203–212. https://doi.org/10.1002/biot.201300195
Ray S, Panjikar S, Anand R (2018) Design of protein-based biosensors for selective detection of benzene groups of pollutants. ACS Sens 3:1632–1638. https://doi.org/10.1021/acssensors.8b00190
Riedel T, Rodriguez-Emmenegger C, de los Santos Pereira A, Bědajánková A, Jinoch P, Boltovets PM, Brynda E (2014) Diagnosis of Epstein–Barr virus infection in clinical serum samples by an SPR biosensor assay. Biosens Bioelectron 55:278–284. https://doi.org/10.1016/j.bios.2013.12.011
Roda A, Guardigli M (2012) Analytical chemiluminescence and bioluminescence: latest achievements and new horizons. Anal Bioanal Chem 402:69–76. https://doi.org/10.1007/s00216-011-5455-8
Roda A, Guardigli M, Calabria D, Calabretta MM, Cevenini L, Michelini E (2014) A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst 139:6494–6501. https://doi.org/10.1039/C4AN01612B
Roda A, Michelini E, Cevenini L, Calabria D, Calabretta MM, Simoni P (2014) Integrating biochemiluminescence detection on smartphones: mobile chemistry platform for point-of-need analysis. Anal Chem 86:7299–7304. https://doi.org/10.1021/ac502137s
Roda A, Mirasoli M, Michelini E, Di Fusco M, Zangheri M, Cevenini L, Roda B, Simoni P (2016) Progress in chemical luminescence-based biosensors: a critical review. Biosens Bioelectron 76:164–179. https://doi.org/10.1016/j.bios.2015.06.017
Rotariu L, Lagarde F, Jaffrezic-Renault N, Bala C (2016) Electrochemical biosensors for fast detection of food contaminants trends and perspective. Trends Anal Chem 79:80–87. https://doi.org/10.1016/j.trac.2015.12.017
Salahandish R, Ghaffarinejad A, Naghib SM, Majidzadeh-A K, Zargartalebi H, Sanati-Nezhad A (2018) Nano-biosensor for highly sensitive detection of HER2 positive breast cancer. Biosens Bioelectron 117:104–111. https://doi.org/10.1016/j.bios.2018.05.043
Sandeau L, Vuillaume C, Contié S, Grinenval E, Belloni F, Rigneault H, Owens RM, Fournet MB (2015) Large area CMOS bio-pixel array for compact high sensitive multiplex biosensing. Lab Chip 15:877–881. https://doi.org/10.1039/C4LC01025F
Sappia L, Felice B, Sanchez MA, Martí M, Madrid R, Pividori MI (2019) Electrochemical sensor for alkaline phosphatase as biomarker for clinical and in vitro applications. Sens Actuators B Chem 281:221–228. https://doi.org/10.1016/j.snb.2018.10.105
Sarkar A, Sarkar KD, Amrutha V, Dutta K (2019) Chapter 15 - An overview of enzyme-based biosensors for environmental monitoring. In: Kaur Brar S, Hegde K, Pachapur VL (eds) Tools, techniques and protocols for monitoring environmental contaminants. Elsevier, pp 307–329
Sasaki JE, Sandroff B, Bamman M, Motl RW (2017) Motion sensors in multiple sclerosis: narrative review and update of applications. Expert Rev Med Devices 14:891–900. https://doi.org/10.1080/17434440.2017.1386550
Satoh T, Kato J, Takiguchi N, Ohtake H, Kuroda A (2004) ATP amplification for ultrasensitive bioluminescence assay: detection of a single bacterial cell. Biosci Biotechnol Biochem 68:1216–1220. https://doi.org/10.1271/bbb.68.1216
Sayhi M, Ouerghi O, Belgacem K, Arbi M, Tepeli Y, Ghram A, Anik Ü, Österlund L, Laouini D, Diouani MF (2018) Electrochemical detection of influenza virus H9N2 based on both immunomagnetic extraction and gold catalysis using an immobilization-free screen printed carbon microelectrode. Biosens Bioelectron 107:170–177. https://doi.org/10.1016/j.bios.2018.02.018
Saylan Y, Denizli A (2018) Molecular fingerprints of hemoglobin on a nanofilm chip. Sensors 18:3016. https://doi.org/10.3390/s18093016
Saylan Y, Erdem Ö, Ünal S, Denizli A (2019) An alternative medical diagnosis method: biosensors for virus detection. Biosensors 9:65. https://doi.org/10.3390/bios9020065
Selvolini G, Marrazza G (2017) MIP-based sensors: promising new tools for cancer biomarker determination. Sensors (Basel) 17. https://doi.org/10.3390/s17040718
Seo G, Lee G, Kim MJ, Baek S-H, Choi M, Ku KB, Lee C-S, Jun S, Park D, Kim HG, Kim S-J, Lee J-O, Kim BT, Park EC, Kim SI (2020) Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 14:5135–5142. https://doi.org/10.1021/acsnano.0c02823
Shafiee H, Lidstone EA, Jahangir M, Inci F, Hanhauser E, Henrich TJ, Kuritzkes DR, Cunningham BT, Demirci U (2014) Nanostructured optical photonic crystal biosensor for HIV viral load measurement. Sci Rep 4:4116. https://doi.org/10.1038/srep04116
Shahar H, Tan LL, Ta GC, Heng LY (2019) Detection of halogenated hydrocarbon pollutants using enzymatic reflectance biosensor. Sens Actuators, B Chem 281:80–89. https://doi.org/10.1016/j.snb.2018.10.076
Sharma AK, Jha R, Gupta BD (2007) Fiber-optic sensors based on surface plasmon resonance: a comprehensive review. IEEE Sens J 7:1118–1129. https://doi.org/10.1109/JSEN.2007.897946
Shim JS, Ahn CH (2012) Optical immunosensor using carbon nanotubes coated with a photovoltaic polymer. Biosens Bioelectron 34:208–214. https://doi.org/10.1016/j.bios.2012.02.004
Singh S, Khajuria R (2020) Utilization of biosensors for environment monitoring. In: Singh J, Vyas A, Wang S, Prasad R (eds) Microbial biotechnology: basic research and applications. Springer, Singapore, pp 299–316
Singh S, Gill AAS, Nlooto M, Karpoormath R (2019) Prostate cancer biomarkers detection using nanoparticles based electrochemical biosensors. Biosens Bioelectron 137:213–221. https://doi.org/10.1016/j.bios.2019.03.065
Soldatkina OV, Soldatkin OO, Kasap BO, Kucherenko DYu, Kucherenko IS, Kurc BA, Dzyadevych SV (2017) A novel amperometric glutamate biosensor based on glutamate oxidase adsorbed on silicalite. Nanoscale Res Lett 12:260. https://doi.org/10.1186/s11671-017-2026-8
Song J, Mauk MG, Hackett BA, Cherry S, Bau HH, Liu C (2016) Instrument-free point-of-care molecular detection of Zika virus. Anal Chem 88:7289–7294. https://doi.org/10.1021/acs.analchem.6b01632
Sousa JB, Ramos-Jesus J, Fonseca RAS, Delerue-Matos C, Barroso MF, Santos JR (2018) Chapter 9 - Biosensors as advanced device for the transgenic plants and food and detection. In: Holban AM, Grumezescu AM (eds) Genetically engineered foods. Academic Press, pp 221–245
Sparaco M, Lavorgna L, Conforti R, Tedeschi G, Bonavita S (2018) The role of wearable devices in multiple sclerosis. Mult Scler Int 2018:7627643. https://doi.org/10.1155/2018/7627643
Subraya KK, Diggs A, Porterfield DM (2013) Amperometric biosensor approaches for quantification of indole 3-acetic acid in plant stress responses. Commun Soil Sci Plant Anal 44:1749–1763. https://doi.org/10.1080/00103624.2013.783920
Sun J-Z, Peter Kingori G, Si R-W, Zhai D-D, Liao Z-H, Sun D-Z, Zheng T, Yong Y-C (2015) Microbial fuel cell-based biosensors for environmental monitoring: a review. Water Sci Technol 71:801–809. https://doi.org/10.2166/wst.2015.035
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians n/a. https://doi.org/10.3322/caac.21660
Tam PD, Van Hieu N, Chien ND, Le A-T, Anh Tuan M (2009) DNA sensor development based on multi-wall carbon nanotubes for label-free influenza virus (type A) detection. J Immunol Methods 350:118–124. https://doi.org/10.1016/j.jim.2009.08.002
Tam YJ, Zeenathul NA, Rezaei MA, Mustafa NH, Azmi MLM, Bahaman AR, Lo SC, Tan JS, Hani H, Rasedee A (2017) Wide dynamic range of surface-plasmon-resonance-based assay for hepatitis B surface antigen antibody optimal detection in comparison with ELISA. Biotechnol Appl Biochem 64:735–744. https://doi.org/10.1002/bab.1528
Tan Y, Wei T (2015) Detection of 17β-estradiol in water samples by a novel double-layer molecularly imprinted film-based biosensor. Talanta 141:279–287. https://doi.org/10.1016/j.talanta.2015.04.019
Tang X, Bansaruntip S, Nakayama N, Yenilmez E, Chang Y, Wang Q (2006) Carbon nanotube DNA sensor and sensing mechanism. Nano Lett 6:1632–1636. https://doi.org/10.1021/nl060613v
Tekaya N, Saiapina O, Ben Ouada H, Lagarde F, Namour P, Ben Ouada H, Jaffrezic-Renault N (2014) Bi-enzymatic conductometric biosensor for detection of heavy metal ions and pesticides in water samples based on enzymatic inhibition in Arthrospira platensis. J Environ Prot 5:441–453. https://doi.org/10.4236/jep.2014.55047
Thouand G, Marks R (2014) Bioluminescence: fundamentals and applications in biotechnology -, vol 2. Springer, Berlin, Heidelberg
Thunemann M, Schmidt K, de Wit C, Han X, Jain RK, Fukumura D, Feil R (2014) Correlative intravital imaging of cGMP signals and vasodilation in mice. Front Physiol 5. https://doi.org/10.3389/fphys.2014.00394
Turemis M, Silletti S, Pezzotti G, Sanchís J, Farré M, Giardi MT (2018) Optical biosensor based on the microalga-paramecium symbiosis for improved marine monitoring. Sens Actuators, B Chem 270:424–432. https://doi.org/10.1016/j.snb.2018.04.111
Vaisocherová-Lísalová H, Víšová I, Ermini ML, Špringer T, Song XC, Mrázek J, Lamačová J, Scott Lynn N, Šedivák P, Homola J (2016) Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens Bioelectron 80:84–90. https://doi.org/10.1016/j.bios.2016.01.040
Verma N, Kaur H, Kumar S (2011) Whole cell based electrochemical biosensor for monitoring lead ions in milk. Biotechnology 10:259–266
Verma N, Singh AK, Kaur P (2015) Biosensor based on ion selective electrode for detection of l-arginine in fruit juices. J Anal Chem 70:1111–1115. https://doi.org/10.1134/S1061934815090129
Verma V, Kala D, Gupta S, Kumar H, Kaushal A, Kuča K, Cruz-Martins N, Kumar D (2021) Leptospira interrogans outer membrane protein-based nanohybrid sensor for the diagnosis of leptospirosis. Sensors 21:2552. https://doi.org/10.3390/s21072552
Vermeir S, Nicolaï BM, Verboven P, Van Gerwen P, Baeten B, Hoflack L, Vulsteke V, Lammertyn J (2007) Microplate differential calorimetric biosensor for ascorbic acid analysis in food and pharmaceuticals. Anal Chem 79:6119–6127. https://doi.org/10.1021/ac070325z
Viter R, Tereshchenko A, Smyntyna V, Ogorodniichuk J, Starodub N, Yakimova R, Khranovskyy V, Ramanavicius A (2017) Toward development of optical biosensors based on photoluminescence of TiO2 nanoparticles for the detection of Salmonella. Sens Actuators, B Chem 252:95–102
Vizzini P, Braidot M, Vidic J, Manzano M (2019) Electrochemical and optical biosensors for the detection of Campylobacter and Listeria: an update look. Micromachines 10:500. https://doi.org/10.3390/mi10080500
Wäckers F, Olson D, Rains G, Lundby F, Haugen J-E (2011) Boar taint detection using parasitoid biosensors. J Food Sci 76:S41-47. https://doi.org/10.1111/j.1750-3841.2010.01887.x
Wang J (2005) Carbon-nanotube based electrochemical biosensors: a review. Electroanalysis 17:7–14. https://doi.org/10.1002/elan.200403113
Wang X, Li Y, Wang H, Fu Q, Peng J, Wang Y, Du J, Zhou Y, Zhan L (2010) Gold nanorod-based localized surface plasmon resonance biosensor for sensitive detection of Hepatitis B virus in buffer, blood serum and plasma. Biosens Bioelectron 26:404–410. https://doi.org/10.1016/j.bios.2010.07.121
Weerathunge P, Ramanathan R, Torok VA, Hodgson K, Xu Y, Goodacre R, Behera BK, Bansal V (2019) Ultrasensitive colorimetric detection of murine norovirus using NanoZyme aptasensor. Anal Chem 91:3270–3276. https://doi.org/10.1021/acs.analchem.8b03300
Weng X, Zhao W, Neethirajan S, Duffield T (2015) Microfluidic biosensor for β-hydroxybutyrate (βHBA) determination of subclinical ketosis diagnosis. J Nanobiotechnology 13. https://doi.org/10.1186/s12951-015-0076-6
Wu L, Deng D, Jin J, Lu X, Chen J (2012) Nanographene-based tyrosinase biosensor for rapid detection of bisphenol A. Biosens Bioelectron 35:193–199. https://doi.org/10.1016/j.bios.2012.02.045
Wu Y, Xue P, Kang Y, Hui KM (2013) Paper-based microfluidic electrochemical immunodevice integrated with nanobioprobes onto graphene film for ultrasensitive multiplexed detection of cancer biomarkers. Anal Chem 85:8661–8668. https://doi.org/10.1021/ac401445a
Wu X, Luo L, Yang S, Ma X, Li Y, Dong C, Tian Y, Zhang L, Shen Z, Wu A (2015) Improved SERS nanoparticles for direct detection of circulating tumor cells in the BLood. ACS Appl Mater Interfaces 7:9965–9971. https://doi.org/10.1021/acsami.5b02276
Xu T, Scafa N, Xu L-P, Zhou S, Al-Ghanem KA, Mahboob S, Fugetsu B, Zhang X (2016) Electrochemical hydrogen sulfide biosensors. Analyst 141:1185–1195. https://doi.org/10.1039/C5AN02208H
Xu Q, Yuan H, Dong X, Zhang Y, Asif M, Dong Z, He W, Ren J, Sun Y, Xiao F (2018) Dual nanoenzyme modified microelectrode based on carbon fiber coated with AuPd alloy nanoparticles decorated graphene quantum dots assembly for electrochemical detection in clinic cancer samples. Biosens Bioelectron 107:153–162. https://doi.org/10.1016/j.bios.2018.02.026
Xu L, Wen Y, Pandit S, Mokkapati VRSS, Mijakovic I, Li Y, Ding M, Ren S, Li W, Liu G (2019) Graphene-based biosensors for the detection of prostate cancer protein biomarkers: a review. BMC Chemistry 13:112. https://doi.org/10.1186/s13065-019-0611-x
Yakoh A, Pimpitak U, Rengpipat S, Hirankarn N, Chailapakul O, Chaiyo S (2021) Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens Bioelectron 176:112912. https://doi.org/10.1016/j.bios.2020.112912
Yang N, Chen X, Ren T, Zhang P, Yang D (2015) Carbon nanotube based biosensors. Sens Actuators B Chem 207:690–715. https://doi.org/10.1016/j.snb.2014.10.040
Yang G, Xiao Z, Tang C, Deng Y, Huang H, He Z (2019) Recent advances in biosensor for detection of lung cancer biomarkers. Biosens Bioelectron 141:111416. https://doi.org/10.1016/j.bios.2019.111416
Yanik AA, Huang M, Kamohara O, Artar A, Geisbert TW, Connor JH, Altug H (2010) An optofluidic nanoplasmonic biosensor for direct detection of live viruses from biological media. Nano Lett 10:4962–4969. https://doi.org/10.1021/nl103025u
Ye W, Xu Y, Zheng L, Zhang Y, Yang M, Sun P (2016) A nanoporous alumina membrane based electrochemical biosensor for histamine determination with biofunctionalized magnetic nanoparticles concentration and signal amplification. Sensors 16:1767. https://doi.org/10.3390/s16101767
Yildirim N, Long F, Gao C, He M, Shi H-C, Gu AZ (2012) Aptamer-based optical biosensor for rapid and sensitive detection of 17β-estradiol in water samples. Environ Sci Technol 46:3288–3294. https://doi.org/10.1021/es203624w
Yoo SM, Lee SY (2016) Optical biosensors for the detection of pathogenic microorganisms. Trends Biotechnol 34:7–25. https://doi.org/10.1016/j.tibtech.2015.09.012
Younis MR, Wang C, Younis MA, Xia X-H (2020) Use of biosensors for mycotoxins analysis in food stuff. In: Nanobiosensors. John Wiley & Sons, Ltd, pp 171–201
Yu H, He Y (2015) Seed-assisted synthesis of dendritic Au–Ag bimetallic nanoparticles with chemiluminescence activity and their application in glucose detection. Sens Actuators, B Chem 209:877–882. https://doi.org/10.1016/j.snb.2014.12.058
Yu F, Tian S, Yao D, Knoll W (2004) Surface plasmon enhanced diffraction for label-free biosensing. Anal Chem 76:3530–3535. https://doi.org/10.1021/ac049964p
Yu Q, Griss R, Schena A, Johnsson K (2017) Chapter Thirteen - Highly modular bioluminescent sensors for small molecules and proteins. In: Thompson RB, Fierke CA (eds) Methods in Enzymology. Academic Press, pp 365–382
Zangheri M, Di Nardo F, Anfossi L, Giovannoli C, Baggiani C, Roda A, Mirasoli M (2015) A multiplex chemiluminescent biosensor for type B-fumonisins and aflatoxin B1 quantitative detection in maize flour. Analyst 140:358–365. https://doi.org/10.1039/c4an01613k
Zare H, Aryan E, Meshkat Z, Gheybi F, Neshani A, Ghazvini K, Rezayi M (2021) Development of biosensors for the detection of COVID-19. Nanomedicine Research Journal 6:11–16. https://doi.org/10.22034/nmrj.2021.01.002
Zehani N, Fortgang P, Saddek Lachgar M, Baraket A, Arab M, Dzyadevych SV, Kherrat R, Jaffrezic-Renault N (2015) Highly sensitive electrochemical biosensor for bisphenol A detection based on a diazonium-functionalized boron-doped diamond electrode modified with a multi-walled carbon nanotube-tyrosinase hybrid film. Biosens Bioelectron 74:830–835. https://doi.org/10.1016/j.bios.2015.07.051
Zeng Y, Zhu Z, Du D, Lin Y (2016) Nanomaterial-based electrochemical biosensors for food safety. J Electroanal Chem 781:147–154. https://doi.org/10.1016/j.jelechem.2016.10.030
Zhang G-J, Zhang L, Huang MJ, Luo ZHH, Tay GKI, Lim E-JA, Kang TG, Chen Y (2010) Silicon nanowire biosensor for highly sensitive and rapid detection of Dengue virus. Sens Actuators B Chem 146:138–144. https://doi.org/10.1016/j.snb.2010.02.021
Zhang HY, Du XY, Liu Q, Xia C, Sun LW (2013) Detection of progesterone in bovine milk using an electrochemical immunosensor. Int J Dairy Technol 66:461–467. https://doi.org/10.1111/1471-0307.12076
Zhang Q, Zhang D, Lu Y, Yao Y, Li S, Liu Q (2015) Graphene oxide-based optical biosensor functionalized with peptides for explosive detection. Biosens Bioelectron 68:494–499. https://doi.org/10.1016/j.bios.2015.01.040
Zhang Z, Sohgawa M, Yamashita K, Noda M (2016) A micromechanical cantilever-based liposome biosensor for characterization of protein-membrane interaction. Electroanalysis 28:620–625. https://doi.org/10.1002/elan.201500412
Zhang W, Liu QX, Guo ZH, Lin JS (2018) Practical application of aptamer-based biosensors in detection of low molecular weight pollutants in water sources. Molecules 23. https://doi.org/10.3390/molecules23020344
Zhou X, Zhu D, Liao Y, Liu W, Liu H, Ma Z, Xing D (2014) Synthesis, labeling and bioanalytical applications of a tris(2,2′-bipyridyl)ruthenium(II)-based electrochemiluminescence probe. Nat Protoc 9:1146–1159. https://doi.org/10.1038/nprot.2014.060
Zhou Z, Xu L, Wu S, Su B (2014) A novel biosensor array with a wheel-like pattern for glucose, lactate and choline based on electrochemiluminescence imaging. Analyst 139:4934–4939. https://doi.org/10.1039/C4AN00687A
Zhu Z, Feng M, Zuo L, Zhu Z, Wang F, Chen L, Li J, Shan G, Luo S-Z (2015) An aptamer based surface plasmon resonance biosensor for the detection of ochratoxin A in wine and peanut oil. Biosens Bioelectron 65:320–326. https://doi.org/10.1016/j.bios.2014.10.059
Zomer G (2010) Chapter 2:The nature of chemiluminescent reactions. In: Chemiluminescence and Bioluminescence. pp 51–90
Acknowledgements
The authors gratefully acknowledge their respective departments/institutes for providing space and other necessary facilities which helped to develop this review article.
Funding
This work was supported by Excellence project Faculty of Science at UHK 2217/2022-2023, by project of Excellence at Faculty of Informatics and management at UHK under ID 2204/2022 and by VT2019-2021.
Author information
Authors and Affiliations
Contributions
U. A., conceptualization, literature survey, table preparation, writing—major original draft, and reference collection; A. K. S. C., literature survey, writing—original draft, and reference collection; P. O., writing—reviewing and editing, figure preparation, reference arrangement, validation, and response; A. M. conceptualization, review and editing, suggestion, response, and supervision; O. K., writing—reviewing and critical editing, response, and supervision; I. H. R., overall reading and reviewing and response; A. D., overall reading and reviewing and response; K. K. review and editing, visualization, response, suggestion, project administration, funding acquisition, and supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This review article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anand, U., Chandel, A.K.S., Oleksak, P. et al. Recent advances in the potential applications of luminescence-based, SPR-based, and carbon-based biosensors. Appl Microbiol Biotechnol 106, 2827–2853 (2022). https://doi.org/10.1007/s00253-022-11901-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11901-6