Abstract
Tenacibaculosis is a major bacterial disease that causes severe fish outbreaks and losses and limits the culture of a variety of commercially valuable anadromous and marine fish species in Europe, America, Asia and Oceania. Fish affected by tenacibaculosis have external lesions and necrosis that affect different areas of the body surface, reducing their commercial value. Several species of Tenacibaculum have been identified as the causal agent of tenacibaculosis in fish, including Tenacibaculum maritimum, Tenacibaculum soleae, Tenacibaculum discolor, Tenacibaculum gallaicum, Tenacibaculum dicentrarchi and “Tenacibaculum finnmarkense” (quotations marks denote species that have not been validly published). Diagnosis of tenacibaculosis is usually based on culture-dependent detection and identification techniques which are time-consuming and do not allow to differentiate closely related species. The development of reliable techniques for studying the relationships between members of the genus Tenacibaculum and for distinguishing fish-pathogenic species of Tenacibaculum genus is, therefore, a key step in understanding the diversity and incidence of tenacibaculosis in global aquaculture, designing effective prevention strategies and early implementation of infection control measures. In this review, recent advances in molecular, serological, proteomic and chemotaxonomic techniques developed for the identification and differentiation of Tenacibaculum species, as well as for the analysis of their genetic and epidemiological relationships are discussed. Key features of current diagnostic methods likely to facilitate control and prevention of tenacibaculosis and to avoid the spread of its aetiological agents are also outlined.
Similar content being viewed by others
Introduction
Tenacibaculosis (formerly marine flexibacteriosis) is an ulcerative bacterial disease responsible for high economic impact in the culture of fish species of commercial interest in Europe, America, Asia, and Oceania (Avendaño-Herrera et al. 2006; Santos et al. 1999; Toranzo et al. 2005). Fish tenacibaculosis is associated with the presence of eroded mouth, necrosis, ulcerate skin lesions, rotted fins, shallow skin lesions, and pale internal organs (Avendaño-Herrera et al. 2006; Santos et al. 1999; Toranzo et al. 2005). Although Tenacibaculum maritimum (formerly Flexibacter maritimus) is considered the main causative agent of tenacibaculosis (Avendaño-Herrera et al. 2006; Santos et al. 1999; Toranzo et al. 2005), other filamentous gliding species belonging to the genus Tenacibaculum, including T. soleae, T. discolor, T. gallaicum, T. dicentrarchi, and “T. finnmarkense” have been isolated from diseased fish and oyster and proved to be virulent under experimental conditions (Avendaño-Herrera et al. 2016; Burioli et al. 2018; Castro et al. 2014a; López et al. 2010; Piñeiro-Vidal et al. 2007, 2008a, b, c; Småge et al. 2016, 2018). The diagnosis of the disease is usually carried out by agar cultivation and biochemical characterization. However, isolation of Tenacibaculum species from fish tissues is difficult due to the slow growth of this bacteria and the overgrowth by other bacteria coexisting in tissue samples. Moreover, identification based on the isolation of the pathogens from fish tissues and their characterization by biochemical tests are time-consuming and do not always allow the differentiation of the Tenacibaculum species pathogenic for fish. The disadvantages associated with conventional diagnostic techniques have prompted the development of alternative identification methods based on the detection of antigens (using antisera raised against the pathogen—serological techniques) or antibodies against the pathogen or nucleic acids (nucleic acid-based techniques), proteins, and fatty acid composition. The objective of this article is to compile the current information on the phenotypic, serological, molecular, and proteomic techniques that have been developed for the detection/identification of the main agents causing tenacibaculosis and the study of their genetic and epidemiological relationships, which have facilitated the early detection and management of this emerging infectious disease.
Fish-associated Tenacibaculum species
The different Tenacibaculum species isolated from fish identified to date, the host species, and geographical distribution are indicated in Table 1.
The first isolation of T. maritimum was reported in cultures of red sea bream Pagrus major and black sea bream Acanthopagrus schlegeli, in Japan (Masumura and Wakabayashi 1977). Since then, T. maritimum has been described as a cause of mortalities in a variety of wild and cultured anadromous and marine fish species worldwide. In Japan, the disease has also been reported in Japanese flounder Paralichthys olivaceous or yellowtail Seriola quinqueradiata (Baxa et al. 1986; Wakabayashi et al. 1986).
In Europe, marine tenacibaculosis is one of the most important and widely spread diseases, affecting the culture of several anadromous and marine fish species. The first report of the existence of T. maritimum in Europe was an outbreak that occurred in Scotland affecting the culture of Dover sole Solea solea (McVicar and White 1979, 1982). Later, the pathogen was identified as the causative agent of disease in the culture of sea bass Dicentrarchus labrax in France (Bernardet et al. 1994; Pépin and Emery 1993). In Spain, marine tenacibaculosis caused by T. maritimum has caused important economic losses in the culture of turbot Scophthalmus maximus, Atlantic salmon Salmo salar, Senegalese sole Solea senegalensis, S. solea, and gilthead sea bream Sparus aurata (Alsina and Blanch 1993; Cepeda and Santos 2002; Devesa et al. 1989; Pazos et al. 1993). In Italy, the disease has been reported in sea bass, sea bream, sharp-snouted bream Diplodus puntazzo, white bream Diplodus sargus, and six-tooted bream Dentex dentex (Salati et al. 2005). Recently, Småge et al. (2016) reported the first isolation of T. maritimum in Norway, isolated from diseased sea lice cleaner Cylopterus lumpus, while Yardimci and Timur (2015) identified T. maritimum as a pathogen of sea bass in Turkey.
In North America, the bacterium has been isolated from white sea bass Atractoscion nobilis, northern anchovy Engraulis mordax, Pacific sardine Sardinops sagax, and Chinook salmon Oncorhynchus tschawytscha (Chen et al. 1995). Frisch et al. (2018) have also identified T. maritimum as the cause of mouth rot infections on the Atlantic salmon aquaculture industry in Western Canada. In South America, T. maritimum has been identified as the causative agent of tenacibaculosis in Chilean Atlantic salmon associated with a Pseudochattonella spp. algal bloom (Apablaza et al. 2017).
In Australia, T. maritimum has been isolated from sea-caged Atlantic salmon and rainbow trout Oncorhynchus mykiss, captured striped trumpeter Latris lineata, greenback flounder Rhombosolea tapirina, yellow-eye mullet Aldrichetta forsteri, and black bream Acanthopagrus butcheri (Handlinger et al. 1997; Soltani and Burke 1994; Soltani et al. 1996).
The fish pathogen T. soleae was first isolated in the Northwest of Spain as the causative agent of marine tenacibaculosis in S. senegalensis by Piñeiro-Vidal et al. (2008b). Later, the pathogen was identified as the cause of mortalities in brill Scophthalmus rhombus and wedge sole Dicologoglossa cuneata (López et al. 2010) and in sea bass (Castro et al. 2014a). Olsen et al. (2017) also isolated T. soleae from cultured wrasse in Norway. Recently, a Flavobacteriaceae strain, closely related to T. soleae isolated from finfish (99% identity with the 16S rRNA sequence of the T. soleae strains present in the GenBank database), was isolated from diseased adult Pacific oysters Crassostrea gigas cultured in Italy (Burioli et al. 2018). The strain expresses pathogenic capacity for infecting Pacific oysters under experimental conditions (Burioli et al. 2018).
The species T. discolor was first isolated from cultured S. senegalensis in the Northwest of Spain (Piñeiro-Vidal et al. 2008a). Since then, this species was isolated from different fish species, including S. maximus, S. senegalensis, and seawater from fish-holding tanks (Piñeiro-Vidal et al. 2008c). In Italy, T. discolor has also been isolated from D. labrax (Habib et al. 2014). The species T. gallaicum has only been isolated in Spain from S. senegalensis, S. maximus, and seawater from fish culture systems (Piñeiro-Vidal et al. 2008a, c).
In 2012, Piñeiro-Vidal et al. described for the first time an outbreak of tenacibaculosis, caused by the pathogen Tenacibaculum dicentrarchi. The bacterium was isolated from skin lesions of diseased D. labrax cultured in Spain. Recently, Avendaño-Herrera et al. (2016) reported the isolation and the negative impact of T. dicentrarchi in the culture of S. salar farmed in Chile. The pathogen has also been isolated from other fish species cultured in Chile, as red conger eel (Irgang et al. 2017). Tenacibaculum-like strains associated with ulcers in cod, wrasse, lump sucker, and Atlantic salmon farmed in Norway were identified as T. dicentrarchi (Olsen et al. 2017).
The last described Tenacibaculum species associated to fish diseases, has been “T. finnmarkense,” which was first reported in cultures of S. salar in Norway (Småge et al. 2016) and recently in Chile (Bridel et al. 2018).
Culture-based diagnostic and identification methods
Diagnosis of tenacibaculosis is usually based on the isolation of the causative agent followed by morphological, biochemical, and serological characterization and analysis of their antimicrobial susceptibility profiles. For primary isolation, routine cultivation, and phenotypic characterization of these seawater-dependent fish-associated Tenacibaculum species, several non-selective, low nutrient media such as Anacker and Ordal agar (AOA) or several modifications, Flexibacter maritimus medium (FMM), TCY (tryptone–casamino acids–yeast extract), tryptone–yeast extract–glucose agar (TYG), and 1/5 Marine Luria Broth (LBM) medium prepared with natural or artificial seawater, have been routinely used (Buller 2014; Pazos et al. 1996; Piñeiro-Vidal 2008; Suzuki et al. 2001). In addition, selective media containing antibiotics have also been tested for the recovery of seawater-dependent flavobacteria (Buller 2014; Pazos et al. 1996), but their use is not very widespread probably because they select resistant strains and do not provide greater bacterial recovery than non-selective media (Pazos et al. 1996). The commercially available Marine agar (MA, 2216E; Difco Laboratory, Detroit, MI, USA) and FMM broth (Laboratorios Conda, Madrid, Spain) media have also been described as suitable for primary isolation of marine gliding bacteria (Buller 2014; Cepeda 2003). For in vitro drug susceptibility testing, FMM agar and broth (Avendaño-Herrera et al. 2005a) and dilute 0.3% Mueller–Hinton Agar (DMHA) or broth prepared with natural or artificial seawater and without or supplemented with 5% fetal calf serum as described by the NCCLS ( 2003) are recommended.
Phenotypic methods based on the study of the biochemical characteristics and antimicrobial susceptibility profiles of bacterial strains remain the most widely used in diagnostic laboratories to identify the causative agent of disease (Austin and Austin 2016; Buller 2014). The phenotypic homogeneity within fish-associated Tenacibaculum species has facilitated their identification based on the analysis of some morphological biochemical and physiological characteristics using traditional microbiological methods and commercially available identification systems such as API ZYM and API 50 CH (Avendaño-Herrera et al. 2006, 2016; Bernardet et al. 2002; Bridel et al. 2018; Irgang et al. 2017; Olsen et al. 2017; Piñeiro-Vidal et al. 2008a, b, c, 2012; Santos et al. 1999; Småge et al. 2016; Suzuki et al. 2001; Toranzo et al. 2005). Table 2 shows the main differential characteristics between the type strains of the fish-associated Tenacibaculum species. Moreover, studies on the susceptibility of the causative agents of tenacibaculosis to different antimicrobial compounds approved for use in aquaculture have demonstrated the existence of variable response depending on the Tenacibaculum species and antimicrobial compound tested. Strains of T. maritimum exhibit a similar pattern, being sensitive to amoxicillin, florfenicol, oxytetracycline, and potentiated sulfonamides (Avendaño-Herrera et al. 2005a, 2008; Pazos 1997; Piñeiro Vidal et al. 2009; Santos et al. 1999). T. gallaicum, T. discolor, T. soleae, and T. dicentrarchi also showed to be susceptible to amoxicillin, florfenicol, and oxytetracycline (Piñeiro Vidal et al. 2009; Piñeiro-Vidal et al. 2012). All these Tenacibaculum species were resistant to oxolinic acid and resistance to flumequine, enrofloxacin, oxytetracycline, and trimethoprim-sulfamethoxazole was also detected in some strains of T. maritimum, T. gallaicum, T. discolor, and T. soleae (Avendaño-Herrera et al. 2008; Piñeiro Vidal et al. 2009). No information is available regarding the susceptibility of “T. finnmarkense” to these antimicrobials.
In addition to biochemical and antibiogram typing, genomic, proteomic, chemotaxonomic, and serological techniques are useful for the differentiation and identification of the different Tenacibaculum species and for describing new taxa of the Family Flavobacteriaceae (Bernardet et al. 2002).
Serological studies and immunological diagnostic methods
Serological techniques are important for diagnosis and epidemiological studies as well as for antigenic characterization to select strains for vaccine formulation. The serological characteristics of T. maritimum were first studied by Wakabayashi et al. (1984) who observed that all the fish isolates tested, regardless of their origin, shared common antigens. This was confirmed in further studies based on whole-cell protein analysis (Avendaño-Herrera et al. 2004a; Bernardet et al. 1994; Pazos et al. 1993; Van Gelderen et al. 2010), agglutination techniques (Baxa et al. 1988; Ostland et al. 1999; Pazos et al. 1993), Dot blot assay (Pazos 1997), immunodiffusion (Baxa et al. 1988; Ostland et al. 1999), and western immunoblots (Pazos 1997; Ostland et al. 1999), using unabsorbed sera against the reference strains NCIMB 2154T and NCIMB 2153 and local strains isolated from different fish species. Later, Baxa et al. (1988) and Pazos (1997) demonstrated the existence of antigenic heterogeneity within T. maritimum using unabsorbed and cross-absorbed sera and established six (Baxa et al. 1988) and four (Pazos 1997) O-serogroups, unrelated with the source of the strains (Table 3). Immunoblot analysis of lipopolysaccharides (LPS) from representative strains of serogroups O1, O2, O3, and O4 isolated from marine and salmonid fish supported the existence of antigenic diversity among T. maritimum isolates and the lack of relationship among the serotype and the source of isolation of the strains (Pazos 1997). Ostland et al. (1999) using LPS immunoblot analysis also described antigenic differences among T. maritimum strains isolated from Atlantic salmon and between these strains and the reference strains (NCIMB 2153, NCIMB 2154T), which may be associated with temporal and geographical aspects. Arenas et al. (2003) using enzyme-linked immunosorbent assay also described the existence of at least three non–host-related serological groups among T. maritimum isolates based on their different reactivity with unabsorbed antisera against the reference strain NCIMB 2153 and one strain isolated from turbot (LPV1.7) (Table 3). The lack of relationship between serotype and host species was also reported by Van Gelderen et al. (2010) who described that the antiserum raised against one strain isolated from Atlantic salmon reacted with the antigens of strains isolated from rainbow trout, Japanese flounder, and red sea bream. Conversely, Avendaño-Herrera et al. (2004a, 2005b) described at least three different O-serogroups of T. maritimum isolated from marine fish which seem to be related to the host species. These authors also described an O-serotyping scheme composed of three major serotypes (O1, O2, and O3) for typing T. maritimum causing mortalities in cultured marine fish (Table 3) (Avendaño-Herrera et al. 2004a, 2005b). Thus, serotype O1 included strains isolated from gilthead sea bream, all the turbot strains belonged to serotype O2, while strains isolated from sole were distributed in serotypes O1 and O3. In a further study, Castro et al. (2007) evaluated the usefulness of this serotyping scheme and demonstrated that serotypes O1 and O3 were also associated with mortalities in blackspot sea bream Pagellus bogaraveo and turbot, respectively. Similarly, Yardimci and Timur (2016) demonstrated that strains isolated from sea bass cultured in Turkey grouped with the serotype O1 defined by Avendaño-Herrera et al. (2004a). To our knowledge, the applicability of this serotyping scheme to identify and type strains isolated from anadromous fish has not yet been evaluated. Moreover, further studies with a high number of strains representative of the worldwide diversity of this pathogen are necessary to elucidate the links of the serogroups with the host fish species and/or geographic area.
The discrepancies regarding the serological groups established for T. maritimum by the different research laboratories could be due to the use of different antigens, antisera, and typing techniques as previously described for this and other filamentous bacteria of the Flavobacteriaceae family (Avendaño-Herrera et al. 2006; Santos et al. 1999; Pazos 1997; Mata et al. 2002; Rochat et al. 2017).
Regarding the serological characteristics of the other fish pathogenic Tenacibaculum species, Piñeiro-Vidal et al. (2006, 2007) using sera against the four serotypes of T. maritimum described by Pazos (1997) and against the type strains of T. soleae, T. discolor, and T. gallaicum demonstrated the existence of antigenic heterogeneity within all these bacterial species and the lack of serological relationship among them. In addition, SDS-PAGE and immunoblot assays of cell envelope (López et al. 2010; Piñeiro-Vidal et al. 2006; Piñeiro-Vidal 2008) and extracellular products (ECP) (Piñeiro-Vidal 2008) corroborated that isolates of the species T. discolor, T. gallaicum, and T. soleae were antigenically different from T. maritimum. This suggests that vaccination against T. maritimum will most likely not protect fish against the other Tenacibaculum fish-associated species. Later, Fernández-Álvarez et al. (2018a) confirmed (1) the lack of cross-reaction between the species T. maritimum, T. soleae, and T. discolor; (2) the existence of at least two serotypes within the species T. soleae; and (3) the existence of at least one serotype for the species T. discolor (Table 3).
Immunological techniques offer simple, rapid, sensitive, and easily automated procedures to confirm disease diagnosis and to detect the pathogens or the antibodies they elicit in the serum of animals as an indicator of previous exposure to the microorganism or infection (González and Santos 2009). Although serological methods are usually employed to evaluate fish immune responses, few efforts have been made to investigate the applicability of serological tests for surveillance purposes and fish health management (Jaramillo et al. 2017). This could be due to the high variability among individuals in their immune responses (Jaramillo et al. 2017) and the difficulty to distinguish between infected and vaccinated fish (Kim et al. 2017).
In contrast, immunological techniques based on the binding between specific antibodies and antigens have been extensively used for the detection and serological characterization of several fish pathogens including members of the genus Tenacibaculum (Avendaño-Herrera et al. 2006; González and Santos 2009; Santos et al. 1999; Toranzo et al. 2005). These techniques are generally faster, more specific, sensitive, and accurate than culture-based diagnostic methods (González and Santos 2009). Nevertheless, the use of methods based on immunological techniques for routine diagnosis of fish tenacibaculosis is hampered by the lack of standardized antisera and/or techniques with a high degree of sensitivity and specificity.
The serological assays more frequently used for the identification of bacterial fish pathogens are based on agglutination reactions such as slide agglutination and microagglutination tests. Early serological studies of T. maritimum (Baxa et al. 1988; Pazos 1997; Wakabayashi et al. 1984) suggested that agglutination-based test may be used for the identification of T. maritimum. However, the problem of auto-agglutinating strains (Avendaño-Herrera et al. 2006; Pazos 1997; Piñeiro-Vidal 2008) together with poor analytical sensitivity has limited their use. Alternatively, dot-blot assay has been described as a fast and inexpensive procedure for the diagnosis of tenacibaculosis as well as for typing T. maritimum, T. soleae, T. gallaicum, and T. discolor strains (Avendaño-Herrera et al. 2004a, 2005b; Castro et al. 2007; Fernández-Álvarez et al. 2018a; Pazos 1997; Piñeiro-Vidal 2008). The sensitivity of this method is like that of ELISA; it has also been used for detection of pathogens in fish tissues (González et al. 2004), but no information is available about the suitability of this technique for the detection of the causative agents of fish tenacibaculosis in field samples.
Immunohistochemistry (IHC) is especially useful for detection of microorganisms that are difficult to identify by microbiological techniques, which are fastidious to grow, or exhibit an atypical morphology (González and Santos 2009). Faílde et al. (2014) designed an IHC-based system for the detection of T. maritimum in paraffin-embedded tissues. The method allowed the specific detection of T. maritimum antigens in several organs of infected Senegalese sole since no cross-reactivity was detected with tissues of fish infected with T. soleae, T. gallaicum, and T. discolor. In addition, the use of IHC allowed the co-location of T. maritimum antigen and a lesion, increasing diagnostic accuracy and understanding of pathogenesis. Yardimci and Timur (2016) used the indirect fluorescent antibody technique (IFAT) for the detection of T. maritimum in fish tissues, but the specificity of this method has not been evaluated. Fluorescent antibody techniques have been previously used to detect this pathogen with contradictory results (Baxa et al. 1988; Powell et al. 2004). IFAT methods are simple, fast, and sensitive; however, they require specialized equipment and skilled operators (González and Santos 2009). Moreover, fluorescence signals can be affected by the antibody quality and concentration and sample preparation method used (Kim et al. 2017).
Recently, Yardimci and Timur (2016) used ELISA and slide agglutination for the detection of antibodies against T. maritimum in the sera of diseased sea bass indicating that these techniques would be more sensitive, rapid, and efficient than the conventional bacteriological diagnostic methods. However, although ELISA techniques are specific and can be automated to increase their time- and cost-effectiveness, they can also yield false-negative results and may exhibit cross-reactivity with closely related antigens (Kim et al. 2017).
The serological diversity of the Tenacibaculum species might have important consequences for the selection of strains for vaccine development, epidemiological surveillance, disease control, and virulence studies. Thus, it would be necessary to develop a common and harmonized serological system and highly sensitive and specific methods for rapid and routine diagnosis and for sero-epidemiological studies of the Tenacibaculum species.
Genotyping and molecular diagnostic methods
Bacterial typing is an important process for diagnosis, treatment, and epidemiological investigation. Various genetic methods have been developed for identification and genotyping of bacterial pathogens. Bacterial genotyping methods are categorized as DNA banding pattern, DNA sequencing, and DNA hybridization. Among them, DNA banding pattern–based methods, which classify the bacteria according to the size of the fragments generated by PCR amplification, by digestion of genomic DNA with restriction enzymes or a combination of both, are the most frequently used for typing pathogenic bacteria (Wolska and Szweda 2012).
Ribotyping has been used for the characterization of several fish pathogenic species including Tenacibaculum maritimum (Farto et al. 1999; Magariños et al. 1997; Pazos 1997). The fragmentation of the DNA from T. maritimum strains using the enzyme Pvu II resulted in the detection of five different rRNA gene restriction patterns (P1 to P5) which were coincident with the four different serotypes described by Pazos (1997) (Table 4). However, this technique was unable to discriminate the strains based on the host source or geographic area of isolation. Considering these results, it would be interesting to analyze the ability of ribotyping as a tool to differentiate T. maritimum from other phenotypically closely related Tenacibaculum species (T. ovolyticum, T. discolor, T. gallaicum, T. dicentrarchi, and “T. finnmarkense”) as well as for the epidemiological characterization of these fish pathogenic species. Although ribotyping has a good reproducibility and discriminatory power at species and subspecies level, it is more expensive and difficult to perform than other typing methods.
In a further study, Avendaño-Herrera et al. (2004d) employed the random amplification polymorphic DNA polymerase chain reaction (RAPD-PCR) technique and revealed the existence of genetic variability within T. maritimum strains isolated from different marine fish. As with the ribotyping technique (Pazos 1997), RAPD analysis revealed molecular patterns coincident with the O-serotypes of T. maritimum. Furthermore, RAPD presented an improvement with respect to ribotyping since the strains were also separated into different groups according to the host species, demonstrating that RAPD is useful for epidemiological studies. RADP analysis also allows to differentiate T. maritimum from T. discolor, T. gallaicum, and T. soleae (Piñeiro-Vidal 2008). However, the discriminatory power of RAPD technique has not been tested with the recently described fish pathogenic species T. dicentrarchi and “T. finnmarkense.” In addition, although this technique is relatively cheap, rapid, and easy to perform, RAPD patterns are notoriously difficult to reproduce from one laboratory to another or when attempting to compare isolates tested on different days.
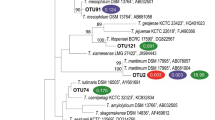
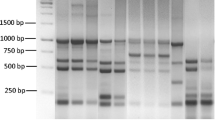
Fernández-Álvarez et al. (2018a) used enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) and repetitive extragenic palindromic PCR (REP-PCR) for typing 68 Tenacibaculum isolates belonging to the species T. maritimum, T. soleae, and T. discolor isolated from several fish species in different geographic areas. ERIC and REP-PCR have proved to be valuable tools to identify and examine diversity among fish-associated Tenacibaculum species since clear different genetic profiles were found for the different Tenacibaculum species analyzed and strains of the same species also displayed distinct genetic patterns (Fernández-Álvarez et al. 2018a). Further analysis including type strains of T. gallaicum, T. dicentrarchi, and T. ovolyticum (Fig. 1) confirmed that both techniques could be used as a diagnostic tool to differentiate Tenacibaculum species (unpublished data from PhD thesis research work of Fernández-Álvarez). Although reproducibility of REP-PCR and ERIC-PCR is much higher than RAPD-PCR due to the specific primers used for amplification, these techniques are not useful for epidemiological purposes since there was a lack of correlation between the genetic profiles of the strains analyzed and their serotype, host source, or geographical area of isolation.
Electrophoretic analysis of type strains of different fish-associated Tenacibaculum species using REP-PCR (a), RAPD (primer 2) (b), and ERIC-PCR (c). MW molecular weight markers; 1—T. maritimum NCIMB 2154T, 2—T. soleae CECT 7292T, 3—T. discolor DSM 18842T, 4—T. dicentrarchi CECT 7612T, 5—T. gallaicum DSM 18841T, 6—T. ovolyticum DSM 18103T
Multi-locus sequence analysis (MLSA) has been successfully used for the genotyping and genetic differentiation of Tenacibaculum species and to estimate the evolutionary relationship among isolates recovered from diseased fish worldwide (Avendaño-Herrera et al. 2016; Frisch et al. 2018; Habib et al. 2014; Olsen et al. 2017). Habib et al. (2014) applied MLSA to a collection of 114 representatives of 18 species of the genus Tenacibaculum, including fish pathogenic and environmental strains. The phylogenetic tree generated by the results of MLSA clearly differentiated T. maritimum, T. soleae, T. gallaicum, T. discolor, T. dicentrarchi, and T. ovolyticum. The study also revealed that pathogenic and environmental lineages are intertwined, indicating that pathogenicity evolved independently in Tenacibaculum species. Moreover, the authors concluded that T. maritimum is a cohesive group consisting of three main genotypes, suggesting that fish health status and environmental conditions, rather than long-distance contamination related to fish movements, are major factors for outbreaks of tenacibaculosis. Olsen et al. (2017) applied the same technique to a collection of 89 Tenacibaculum isolates associated with ulcers in sea-farmed fish in Norway and identified the strains as T. maritimum, T. dicentrarchi, T. soleae, and T. ovolyticum. MLSA-based phylogenetic studies showed that Canadian isolates of T. maritimum recovered from Atlantic salmon were closely related to strains isolated in Norway and Chile and separated from other Tenacibaculum type strains (Frisch et al. 2018). Similarly, Avendaño-Herrera et al. (2016) applied MLSA to report the first isolation of T. dicentrarchi from Atlantic salmon in Chile and to reveal the close relationship between the Chilean isolates and the T. dicentrarchi isolates recovered from cod in Norway. Overall, MLSA seems to be a reliable technique for the differentiation of Tenacibaculum species and for epidemiological studies and could be of help in monitoring tenacibaculosis in marine aquaculture. In addition, MLSA provides unambiguous DNA sequence data that can be easily exchanged and compared via web-based databases around the world and combines PCR and automated DNA sequencing to reduce labor and analysis time, thereby providing discriminatory power comparable or superior to that provided by other PCR-based methods (Lin et al. 2014).
Regarding molecular diagnostic methods, considerable efforts have been made in recent years to adapt PCR to the detection of fish pathogens in aquaculture and to discriminate between non-pathogenic and pathogenic strains (Buller 2014; Toranzo et al. 2005). These tests have advantages over conventional methods, such as sensitivity, specificity, and accuracy, allowing the identification of non-cultivable or slow-growing microorganisms. Moreover, the high sensitivity of PCR allows the detection of pathogens shortly after infection and even before the onset of the disease, which facilitates the early establishment of specific treatments and management of the disease.
Different PCR methods have been designed for the detection and identification of Tenacibaculum species pathogenic for fish both from pure or mixed cultures and/or fish tissues (Avendaño-Herrera et al. 2004b, c; Avendaño-Herrera et al. 2017; Bader and Shotts 1998; Castro et al. 2014b; Cepeda et al. 2003; Fernández-Álvarez et al. 2019; Fringuelli et al. 2012; García-González et al. 2011; López et al. 2011; Småge et al. 2017, 2018; Toyama et al. 1996; Warsen et al. 2004; Wilson et al. 2002; Wilson and Carson 2003). Many of the PCR protocols described for the detection of these bacteria are based on the amplification of ribosomal genes, mainly 16S.
Table 5 shows the available primers and procedures for specific PCR detection of Tenacibaculum species causing pathological problems in aquaculture. For the detection of T. maritimum, two different primer pairs targeting the 16S rRNA gene have been designed. Thus, Toyama et al. (1996) developed the primer pair MAR1 and MAR2 that amplified a specific fragment of 1088 bp of the 16S rRNA gene of T. maritimum and allowed to differentiate this species from other fish pathogenic flavobacteria. Later, Bader and Shotts (1998) designed a new pair of primers Mar1 and Mar2 that flanked a fragment of 400 bp of the 16S rRNA gene of T. maritimum. Both methods have successfully been used to identify pure and mixed cultures of T. maritimum (Avendaño-Herrera et al. 2004b, c; Cepeda and Santos 2002; Cepeda et al. 2003; Småge et al. 2016). The procedure designed by Bader and Shotts (1998) also allowed to distinguish T. maritimum from other taxonomically related fish pathogenic Tenacibaculum species (T. discolor, T. gallaicum, and T. soleae) (Piñeiro-Vidal et al. 2007; Piñeiro-Vidal 2008). However, these simple PCR protocols presented low sensitivity for detecting low levels of bacteria in fish samples (Cepeda and Santos 2002; Cepeda et al. 2003; Avendaño-Herrera et al. 2006); therefore, different nested-PCR approaches were developed. Our group (Cepeda and Santos 2002; Cepeda et al. 2003) described the first nested-PCR protocol for the detection of T. maritimum using tissue samples recovered from fish suffering from tenacibaculosis. The method, based on a first amplification using the universal primers 20F and 1500R (Weisburg et al. 1991) and a second PCR using the species-specific primers described by Bader and Shotts (1998), proved to be extremely sensitive (capable of detecting 75 CFU mg−1 fish tissue). Moreover, the method allowed differentiating T. maritimum strains from Flavobacterium species and non-related pathogens. Later, Avendaño-Herrera et al. (2004c) developed another nested-PCR protocol based on a first round of PCR using the universal primer pair pA and pH (Edwards et al. 1989) and a second round using the species-specific primers MAR1 and MAR2 designed by Toyama et al. (1996). This protocol proved to be highly specific since no amplification was detected when other Tenacibaculum-like strains, T. mesophilum, T. ovolyticum, T. amylolyticum as well as non-related pathogens were tested. Although methods based on nested PCR are more sensitive than single-step PCR, they are also more expensive and time-consuming since it requires two rounds of PCR, with the possible risk of amplicon contamination, and the analysis of the amplification products by agarose gel electrophoresis. Other PCR-based methods devised for the diagnosis of fish tenacibaculosis include PCR–enzyme-linked immunosorbent assay (PCR-ELISA) (Wilson et al. 2002), reverse transcriptase polymerase chain reaction–enzyme hybridisation assay (RT-PCR-EHA) (Wilson and Carson 2003), and DNA microarray probe (Warsen et al. 2004). These assays have shown enough sensitivity for the direct detection of T. maritimum from pure culture, but their effectiveness for detection of the bacterium in fish tissues or environmental samples and their capacity to differentiate fish pathogenic Tenacibaculum species has not been evaluated.
Quantitative real-time PCR tests have been extensively developed in clinical microbiology laboratories for routine diagnosis of infectious diseases, particularly bacterial diseases. This molecular tool is well suited for the rapid detection of bacteria directly in clinical specimens, allowing early, sensitive, and specific laboratory confirmation of diseases. Fringuelli et al. (2012) developed a quantitative real-time PCR using a set of primers and a Taqman probe to amplify a 155-bp fragment of the 16SrRNA gene of T. maritimum for its detection in field samples (Table 5). Although the test proved to be highly sensitive (4.8 DNA copies number/μl), the specificity of the assay was only tested in silico. Thus, it would be interesting to test the specificity in vitro using other Tenacibaculum species pathogenic for fish. This protocol was also able to detect T. maritimum in formalin-fixed paraffin-embedded materials with a high degree of sensitivity. The implementation of this trial could represent an important step toward a rapid and safe diagnosis of tenacibaculosis and provides a useful tool for elucidating important disease processes.
Recently, Fernández-Álvarez et al. (2019) described a real-time PCR assay coupled with melting curve analysis targeting a fragment of 164 bp of the sequence of the 16S rRNA gene for the detection and quantification of T. maritimum. The method allows the identification of T. maritimum bacterial cultures, and the rapid, sensitive (2.22 amplicon copies number/μl), and specific detection and quantification of the bacterium in lethal and non-lethal fish samples and seawater samples. Thus, this protocol could be used for epidemiological studies, monitoring the presence of T. maritimum in fish production systems, and to evaluate the quality of waters entering and leaving the aquaculture facilities, which may help to prevent the occurrence of tenacibaculosis outbreaks.
For the specific detection of the fish pathogen T. soleae, two primer sets were described in 2011 (García-González et al. 2011; López et al. 2011) (Table 5). García-González et al. (2011) designed a simple PCR protocol based on the amplification of an internal fragment of 248 bp of the 16S rRNA gene. On the other hand, López et al. (2011) designed a set of primers targeting the 16S rRNA gene and the internal spacer region (ISR), yielding a 1555 bp fragment. Both nucleic acid-based methods were highly specific and sensitive, differentiating T. soleae from other Tenacibaculum species as well as from several non-related bacterial pathogens. Moreover, the assay developed by López et al. (2011) proved to be more sensitive than agar cultivation for the detection of T. soleae from naturally diseased fish, offering a useful tool for diagnosis and for understanding the epidemiology of this pathogen.
PCR-based procedures have also been described for identification and detection of the most recently described Tenacibaculum species associated to fish diseases. A molecular approach based on simple PCR was recently developed (Avendaño-Herrera et al. 2017) for the specific identification and detection of the fish pathogen T. dicentrarchi, using the primers Tenadi Fw and Tenadi Rv, yielding an amplicon of 284 bp of the 16S rRNA gene (Table 5). Moreover, Småge et al. (2017) described that Tb_tuf real-time RT-PCR procedure (Vold 2014) that uses a primer pair and a probe for the amplification of the tuf gene, which encodes the elongation factor Tu, allows the detection of Tenacibaculum spp. in skin tissues of Atlantic salmon. Sequencing and phylogenetic analysis allowed confirming that most isolates recovered from the diseased fish belonged to “T. finnmarkense” species. Further, Småge et al. (2018) described a new real-time RT-PCR assay using as target the housekeeping gene rpoB (Tb_rpoB assay). This assay detects the common Tenacibaculum spp. strains isolated from diseased salmon in Northern Norway and T. soleae while the Tb_tuf assay was less specific detecting a wider range of Tenacibaculum spp. including T. ovolyticum and T. soleae (Småge et al. 2018).
Future works should focus on the development of specific PCR-based methods for the diagnosis of tenacibaculosis caused by “T. finnmarkense,” T. discolor, T. gallaicum, and T. ovolyticum. Indeed, co-infections of the same individuals by two different Tenacibaculum species or by Tenacibaculum species and other fish-pathogenic species have been reported (Cepeda 2003; Piñeiro-Vidal 2008; Santos et al. 1999; Toranzo et al. 2005) and tests that allow to reveal this are desirable. In addition, the development of multiplex PCR protocols, which allows the detection of multiple targets (pathogens) in a single sample, will increase the testing capacity and reduce the amount of time and consumables used in the process, thus reducing the overall costs of the test. In this sense, López et al. (2012) developed a different approach based on reverse line blot hybridization (RLB), which relies on the hybridization of the PCR products to specific probes to identify differences in the amplified sequences. The authors designed an RLB assay using species-specific probes targeting the 16S–23S ISR or the 23S rRNA gene. This technique enabled the simultaneous identification of different fish pathogens, including T. maritimum, T. soleae, Vibrio harveyi, Photobacterium damselae, and Pseudomonas baetica and their differentiation from other non-target bacteria. Later, Castro et al. (2014b) designed a multiplex PCR protocol using the primers designed by Toyama et al. (1996) and Sakai et al. (2007) for the simultaneous detection of T. maritimum and Edwardsiella tarda. The authors did not observe non-specific amplifications using DNA from other related bacteria including T. soleae, T. discolor, and T. gallaicum.
Knowledge of the complete genome sequence of Tenacibaculum species associated to fish diseases will help to improve diagnostics and better understand the biology of the Tenacibaculum genus. The complete genome sequence of T. maritimum, T. ovolyticum, T. dicentrarchi, and T. soleae (Grothusen et al. 2016; Lujan et al. 2016; Pérez- Pascual et al. 2017; Teramoto et al. 2016) is now available.
Further studies should be focused on genome-wide comparative studies to identify key molecular determinants of virulence, antimicrobial resistance, and serotypes of T. maritimum and the other fish-associated Tenacibaculum species which will allow the design of specific primers and PCR protocols. Several multiplex-PCR protocols have been recently developed for the identification of serotypes of different bacterial species such as Flavobacterium psychrophilum (Rochat et al. 2017) or Streptococcus parauberis (Tu et al. 2015; Torres-Corral et al. 2019).
PCR-based diagnostic approach combining the detection of pathogens, their mechanisms of antibiotic resistance, their virulence factors, and bacterial load in samples of diseased fish could lead to profound changes in the management of tenacibaculosis.
Proteomic and chemotaxonomic methods as a tool for diagnosis and epidemiological studies
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) has emerged as a powerful tool for microbial identification, strain typing, epidemiological studies, or detection of antibiotic resistance (Singhal et al. 2015). The MALDI-TOF-MS methodology typically targets the mass range 2000 to 20,000 Da, including ribosomal proteins and other housekeeping proteins and structural proteins that are abundant in the cell and relatively independent of growth state and external stimuli (Welker 2011). The ability of the MALDI-TOF-MS technique to produce comparable spectra using different media is important for the construction of spectra databases that can be used as a reliable tool for the rapid classification and identification of the unknown bacterial isolates in diagnostic laboratories all over the world. In recent years, great efforts have been carried out to construct protein databases for the identification and for the study of phylo-proteomic relationships between closely related fish bacterial pathogens (Fernández-Álvarez et al. 2016, 2017, 2018a, 2018b; Pérez-Sancho et al. 2016, 2017; Assis et al. 2017; López-Cortés et al. 2017). Fernández-Álvarez et al. (2017) applied for the first time the technique MALDI-TOF-MS on Tenacibaculum isolates, including the species T. maritimum, T. soleae, T. discolor, T. gallaicum, T. dicentrarchi, and T. ovolyticum. The authors identified eight genus-specific peaks (m/z 2273.50; 2433.48; 2661.82; 4789.03; 5048.15; 5314.34; 10,507.39; 13,148.28) that could be used as biomarkers for rapid diagnosis of tenacibaculosis, and to differentiate the species of this genus from other marine fish pathogens. The spectra of all the species analyzed showed a high number of peaks in common. However, species-specific mass peaks were found for the species T. maritimum, T. soleae, T. ovolyticum, and T. dicentrarchi that allowed their differentiation and specific identification of these species (Table 6). However, mass spectra from the species T. gallaicum and T. discolor were highly similar, and no species-specific peaks could be detected in this study. Despite the importance of reference databases for identification, MALDI-TOF has also proven valuable for the characterization of microorganisms for which no reference mass spectra exist, using cluster analysis of proteomic mass data (Conway et al. 2001; Böhme et al. 2011). Cluster and principal component analysis (PCA) of the mass data also proved to be reliable methods for taxonomy of Tenacibaculum species by comparing similarities and differences in their proteomic profiles, except for the species T. discolor and T. gallaicum (Fernández-Álvarez et al. 2017). In a later study, Fernández-Álvarez et al. (2018a) used the MALDI-TOF-MS for the identification of the different serotypes described for the species T. maritimum, T. soleae, and T. discolor. Although no serotype-specific mass peaks were found for the different serotypes of T. maritimum, probably due to the low number of strains belonging to serotypes O2, O3, and O4 used, specific peaks were found for the species T. soleae and T. discolor. In total, 11 serotype-specific mass peaks were found for the serotype O1 of T. soleae and 8 mass peaks were present in all the T. soleae strains belonging to serotype O2. For the species T. discolor, a comparative analysis of spectra revealed the existence of two specific mass peaks for the O1 serotype (Fernández-Álvarez et al. 2018a). Further studies with a high number of strains representatives of pathogenic Tenacibaculum species with different hosts, sources are needed to evaluate the capacity of MALDI-TOF for epidemiological studies and to confirm its efficacy as diagnostic method.
Chemotaxonomic methods allow classifying microorganisms based on differences and similarities in chemical markers (cell wall constituents such as fatty acids, polar lipids, lipopolysaccharide…). Currently, these approaches have been successfully established as routine identification methods, together with classical biochemical tests, serological assays, and genome sequencing. Moreover, Bernardet et al. (2002) included the presence or amount of some fatty acids as a characteristic for describing new taxa of the family Flavobacteriaceae and to differentiate a new taxon from existing taxa. This chemotaxonomic tool had been successfully applied in taxonomic studies for the identification of other fish pathogenic species such as F. columnare (Shoemaker et al. 2005). The determination of the fatty acid methyl esters content was used for taxonomic and epidemiological characterization of the species T. maritimum, T. discolor, T. gallaicum, T. soleae, T. dicentrarchi, and T. ovolyticum (Piñeiro-Vidal 2008; Piñeiro-Vidal et al. 2008a, b, c, 2012). All the species analyzed were characterized by the presence of large amounts of branched and hydroxylated fatty acids. However, results revealed the existence of differences in the fatty acids profiles between the T. maritimum strains isolated from different marine fish or geographical origin, as well as between strains of T. maritimum, T. discolor, T. gallaicum, T. soleae, T. dicentrarchi, and T. ovolyticum (Table 7). Thus, T. maritimum, T. discolor, T. gallaicum, and T. ovolyticum contained 32, 37, 34, and 36 different fatty acids, respectively. FAMEs from the four species significantly differed in the content of iso-C15:03-OH, iso-C16:03-OH, iso-C15:1 G, summed feature 3, iso-C16:0, C17:1ω6c, C15:03-OH, and iso-C17:03-OH. The main difference between T. soleae and the other Tenacibaculum species was the high content (13.9%) of unsaturated fatty acids (C15:1ω6c and C17:1ω6c) (Piñeiro-Vidal et al. 2008c). These differences in the fatty acids profiles allowed to evaluate the contribution of each fatty acid in the differentiation of strains of T. maritimum, T. discolor, T. gallaicum, T. soleae, and T. ovolyticum analyzed and to determine the taxonomic relationships between these strains (Piñeiro-Vidal 2008; Piñeiro-Vidal et al. 2008c). Further studies identified the major fatty acids for the type strain of T. dicentrarchi (Piñeiro-Vidal et al. 2012) and “T. finnmarkense” (Småge et al. 2016). The cellular fatty acid profile of T. dicentrarchi was dominated by iso-C15:0, iso-C15:03-OH, anteiso C15:0, C15:1ω6c, and iso-C15:1. The major cellular fatty acids (> 5% of the total fatty acids) of the type strain of “T. finnmarkense” are summed feature 3 (comprising C16:1ω7c and/or iso-C15:02-OH), iso C15:0, anteiso-C15:0, iso-C15:1, and iso-C15:0 3-OH. Table 7 shows the characteristic fatty acids of fish-associated Tenacibaculum species. Although necessary for the description of new bacterial species, the use of fatty acid analysis for routine identification of Tenacibaculum is limited because the results obtained depend largely on growth conditions and the procedure is expensive compared to other available genomic and proteomic procedures.
In conclusion, both phenotypic and molecular approaches are useful for the diagnosis of tenacibaculosis. The harmonization of serotyping systems and the development of a common molecular diagnostic system, with an appropriate detection rate, accuracy, and reproducibility to ensure compatibility of results between laboratories, will help in understanding the diversity and incidence of tenacibaculosis in global aquaculture, designing effective prevention strategies and early implementation of infection control measures. Moreover, DNA-based strain typing techniques are a remarkably useful set of tools for epidemiological studies, among them MLSA could be considered the reference method for typing bacterial pathogens of the Tenacibaculum genus.
References
Alsina M, Blanch AR (1993) First isolation of Flexibacter maritimus from cultivated turbot (Scophthalmus maximus). Bull Eur Assoc Fish Pathol 13(5):157–160
Apablaza P, Frisch K, Brevik ØJ, Småge SB, Vallestad C, Duesund H, Mendoza J, Nylund A (2017) Primary isolation and characterization of Tenacibaculum maritimum from Chilean Atlantic salmon mortalities associated with a Pseudochattonella spp. algal bloom. J Aquat Anim Health 29(3):143–149
Arenas J, Mata M, Santos Y (2003) Evaluation of an enzyme-linked immunosorbent assay for serological typing of Tenacibaculum maritimum. European Association of Fish Pathologist 11th International Conference of ‘Disease of Fish and Shell Fish’
Assis GB, Pereira FL, Zegarra AU, Tavares GC, Leal CA, Figueiredo HC (2017) Use of MALDI-TOF mass spectrometry for the fast identification of gram-positive fish pathogens. Front Microbiol 8:1–12
Austin B, Austin DA (2016) Bacterial fish pathogens, 6th edn. Springer, Chichester
Avendaño-Herrera R, Magariños B, López-Romalde S, Romalde JL, Toranzo AE (2004a) Phenotypic characterization and description of two major O-serotypes in Tenacibaculum maritimum strains from marine fish. Dis Aquat Org 58:1–8
Avendaño-Herrera R, Núñez S, Magariños B, Toranzo AE (2004b) A non-destructive method for rapid detection of Tenacibaculum maritimum in farmed fish using nested PCR amplification. Bull Eur Assoc Fish Pathol 24(6):280–286
Avendaño-Herrera R, Magariños B, Toranzo AE, Beaz R, Romalde JL (2004c) Species-specific polymerase chain reaction primer sets for the diagnosis of Tenacibaculum maritimum infection. Dis Aquat Org 62:75–83
Avendaño-Herrera R, Rodríguez J, Magariños B, Romalde JL, Toranzo AE (2004d) Intraspecific diversity of the marine fish pathogen Tenacibaculum maritimum as determined by randomly amplified polymorphic DNA-PCR. J Appl Microbiol 96(4):871–877
Avendaño-Herrera R, Irgang R, Núñez S, Romalde JL, Toranzo AE (2005a) Recommendation of an appropriate medium for in vitro drug susceptibility testing of the fish pathogen Tenacibaculum maritimum. Antimicrob Agents Chemother 49:82–87
Avendaño-Herrera R, Magariños B, Moriñigo MA, Romalde JL, Toranzo AE (2005b) A novel O-serotype in Tenacibaculum maritimum strains isolated from cultured sole (Solea senegalensis). Bull Eur Assoc Fish Pathol 25(2):70–74
Avendaño-Herrera R, Toranzo AE, Magariños B (2006) Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: a review. Dis Aquat Org 71:255–266
Avendaño-Herrera R, Nuñez S, Barja JL, Toranzo AE (2008) Evolution of drug resistance and minimum inhibitory concentration to enrofloxacin in Tenacibaculum maritimum strains isolated in fish farms. Aquac Int 16(1):1–11
Avendaño-Herrera R, Irgang R, Sandoval C, Moreno-Lira P, Houel A, Duchaud E, Poblete-Morales M, Nicolas P, Ilardi P (2016) Isolation, characterization and virulence potential of Tenacibaculum dicentrarchi in salmonid cultures in Chile. Transbound Emerg Dis 63(2):121–126
Avendaño-Herrera R, Irgang R, Tapia-Cammas D (2017) PCR procedure for detecting the fish pathogen Tenacibaculum dicentrarchi. J Fish Dis 41(4):715–719
Bader JA, Shotts EB (1998) Identification of Flavobacterium and Flexibacter species by species-specific polymerase chain reaction primers to the 16S ribosomal RNA gene. J Aquat Anim Health 10(4):311–319
Baxa DV, Kawai K, Kusuda R (1986) Characteristics of gliding bacteria isolated from diseased cultured flounder, Paralichthys olivaceous. Fish Pathol 21(4):251–258
Baxa DV, Kawai K, Kusuda R (1988) Detection of Flexibacter maritimus by fluorescent antibody technique in experimentally infected black sea bream fry. Fish Pathol 23(1):29–32
Bernardet JF, Campbell AC, Buswell JA (1990) Flexibacter maritimus is the agent of “black patch necrosis” in Dover sole in Scotland. Dis Aquat Org 8:233–237
Bernardet JF, Kerouault B, Michel C (1994) Comparative study on Flexibacter maritimus strains isolated from farmed sea bass (Dicentrarchus labrax) in France. Fish Pathol 29(2):105–111
Bernardet JF, Nakagawa Y, Holmes B (2002) Proposed minimal standards for describing new taxa of the family Flavobacteriaceae and emended description of the family. Int J Syst Evol Microbiol 52:1049–1070
Böhme K, Fernández-No IC, Barros-Velázquez J, Gallardo JM, Cañas B, Calo-Mata P (2011) Rapid species identification of seafood spoilage and pathogenic gram-positive bacteria by MALDI-TOF mass fingerprinting. Electrophoresis 32(21):2951–2965
Bridel S, Olsen AB, Nilsen H, Bernardet JF, Achaz G, Avendaño-Herrera R, Duchaud E (2018) Comparative genomics of Tenacibaculum dicentrarchi and “Tenacibaculum finnmarkense” highlights intricate evolution of fish-pathogenic species. Genome Biol Evol 10(2):452–457
Buller NB (2014) In: Buller NB (ed) Bacteria and fungi from fish and other aquatic animals: a practical identification manual, 2nd edn. Cabi Publishing, Wallingford
Burioli EAV, Varello K, Trancart S, Bozzetta E, Gorla A, Prearo M, Houssin M (2018) First description of a mortality event in adult Pacific oysters in Italy associated with infection by a Tenacibaculum soleae strain. J Fish Dis 41(2):215–221
Castro N, Magariños B, Núñez S, Toranzo AE (2007) Reassessment of the Tenacibaculum maritimum serotypes causing mortalities in cultured marine fish. Bull Eur Assoc Fish Pathol 27(6):229–233
Castro N, Balboa S, Núñez S, Toranzo AE, Magariños B (2014a) First isolation and characterization of Tenacibaculum soleae from sea bass Dicentrarchus labrax. Fish Pathol 49(1):16–22
Castro N, Toranzo AE, Magariños B (2014b) A multiplex PCR for the simultaneous detection of Tenacibaculum maritimum and Edwardsiella tarda in aquaculture. Int Microbiol 17:111–117
Cepeda C (2003) Desarrollo de sistemas eficaces para el cultivo y la identificación de Flavobacterium psychrophilum y Tenacibaculum maritimum. Tesis doctoral, Universidad Santiago de Compostela (Santiago de Compostela)
Cepeda C, Santos Y (2002) First isolation of Flexibacter maritimus from farmed Senegalese sole (Solea senegalensis, Kaup) in Spain. Bull Eur Assoc Fish Pathol 22(6):388–392
Cepeda C, García-Márquez S, Santos Y (2003) Detection of Flexibacter maritimus in fish tissue using nested PCR amplification. J Fish Dis 26(2):65–70
Chen ME, Henry-Ford D, Groff JM (1995) Isolation and characterization of Flexibacter maritimus from marine fishes of California. J Aquat Anim Health 7(4):318–326
Conway GC, Smole SC, Sarracino DA, Arbeit RD, Leopold PE (2001) Phyloproteomics: species identification of Enterobacteriaceae using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Mol Microbiol Biotechnol 3(1):103–112
Devesa S, Barja JL, Toranzo AE (1989) Ulcerative skin and fin lesions in reared turbot, Scophthalmus maximus (L). J Fish Dis 12(4):323–333
Edwards U, Rogall T, Bloecker H, Emde M, Boettger EC (1989) Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17(19):7843–7853
Faílde LD, Bermúdez R, Losada AP, Riaza A, Santos Y, Quiroga MI (2014) Immunohistochemical diagnosis of tenacibaculosis in paraffin-embedded tissues of Senegalese sole Solea senegalensis Kaup, 1858. J Fish Dis 37(11):959–968
Farto R, Montes M, Pérez MJ, Nieto TP, Larsen JL, Pedersen K (1999) Characterization by numerical taxonomy and ribotyping of Vibrio splendidus biovar I and Vibrio scophthalmi strains associated with turbot cultures. J Appl Microbiol 86(5):796–804
Fernández-Álvarez C, Gijón D, Álvarez M, Santos Y (2016) First isolation of Aeromonas salmonicida subsp. salmonicida from diseased sea bass, Dicentrarchus labrax (L.), cultured in Spain. Aquac Rep 4:36–41
Fernández-Álvarez C, Torres-Corral Y, Saltos-Rosero N, Santos Y (2017) MALDI-TOF mass spectrometry for rapid differentiation of Tenacibaculum species pathogenic for fish. Appl Microbiol Biotechnol 101(13):5377–5390
Fernández-Álvarez C, Torres-Corral Y, Santos Y (2018a) Comparison of serological and molecular typing methods for epidemiological investigation of Tenacibaculum species pathogenic for fish. Appl Microbiol Biotechnol 102(6):2779–2789
Fernández-Álvarez C, Torres-Corral Y, Santos Y (2018b) Use of ribosomal proteins as biomarkers for identification of Flavobacterium psychrophilum by MALDI-TOF mass spectrometry. J Proteome 170:59–69
Fernández-Álvarez C, González SF, Santos Y (2019) Quantitative PCR coupled with melting curve analysis for rapid detection and quantification of Tenacibaculum maritimum in fish and environmental samples. Aquaculture 498(1):289–296
Florio D, Gridelli S, Fioravanti ML, Zanoni RG (2016) First isolation of Tenacibaculum maritimum in a captive sand tiger shark (Carcharias taurus). J Zoo Wildl Med 47(1):351–353
Fringuelli E, Savage PD, Gordon A, Baxter EJ, Rodger HD, Graham DA (2012) Development of a quantitative real-time PCR for the detection of Tenacibaculum maritimum and its application to field samples. J Fish Dis 35(8):579–590
Frisch K, Småge SB, Brevik ØJ, Duesund H, Nylund A (2018) Genotyping of Tenacibaculum maritimum isolates from farmed Atlantic salmon in Western Canada. J Fish Dis 41(1):131–137
García-González P, García-Lamas N, Fuentes Edfuf C, Santos Y (2011) Development of a PCR method for the specific identification of the marine fish pathogen Tenacibaculum soleae. Aquaculture 319:1–4
González SF, Santos Y (2009) Serological methods for the detection of pathogenic bacteria in aquaculture: present status and prospects. In: Fisheries, aquaculture and biotechnology. Agrobios, India, pp 131–144
González SF, Osorio CR, Santos Y (2004) Evaluation of the AQUARAPID-Va, AQUAEIA-Va and dot-blot assays for the detection of Vibrio anguillarum in fish tissues. J Fish Dis 27:617–621
Grothusen H, Castillo A, Henríquez P, Navas E, Bohle H, Araya C, Bustamante F, Bustos P, Mancilla M (2016) First complete genome sequence of Tenacibaculum dicentrarchi, an emerging bacterial pathogen of salmonids. Genome Announc 4(1):e01756–e01715. https://doi.org/10.1128/genomeA.01756-15
Habib C, Houel A, Lunazzi A, Bernardet JF, Olsen AB, Nilsen H, Toranzo AE, Castro N, Nicolas P, Duchaud E (2014) Multilocus sequence analysis of the marine bacterial genus Tenacibaculum suggests parallel evolution of fish pathogenicity and endemic colonization of aquaculture systems. Appl Environ Microbiol 80(17):5503–5514
Handlinger J, Soltani M, Percival S (1997) The pathology of Flexibacter maritimus in aquaculture species in Tasmania, Australia. J Fish Dis 20(3):159–168
Hansen GH, Bergh Ø, Michaelsen J, Knappskog D (1992) Flexibacter ovolyticus sp. nov. a pathogen of eggs and larvae of Atlantic halibut, Hipoglossus hipoglossus L. Int J Syst Bacteriol 42:451–458
Irgang R, González-Luna R, Gutiérrez J, Poblete-Morales M, Rojas V, Tapia-Cammas D, Avendaño-Herrera R (2017) First identification and characterization of Tenacibaculum dicentrarchi isolated from Chilean red conger eel (Genypterus chilensis, Guichenot 1848). J Fish Dis 40(12):1915–1920
Jaramillo D, Peeler EJ, Laurin E, Gardner IA, Whittington RJ (2017) Serology in finfish for diagnosis, surveillance, and research: a systematic review. J Aquat Anim Health 29:1–14
Kim A, Nguyen TL, Kim DH (2017) Modern methods of diagnosis. In: Austin B, Newaj-Fyzul A (eds) Diagnosis and control of diseases of fish and shellfish. John Wiley & Sons Ltd, Hoboken, pp 109–145
Kolygas MN, Gourzioti E, Vatsos IN, Athanassopoulou F (2012) Identification of Tenacibaculum maritimum strains from marine farmed fish in Greece. Vet Rec 170:623
Lin T, Lin L, Zhang F (2014) Review on molecular typing methods of pathogens. Open J Med Microbiol 4:147–152
López JR, Núñez S, Magariños B, Castro N, Navas JI, De La Herran R, Toranzo AE (2009) First isolation of Tenacibaculum maritimum from wedge sole, Dicologoglossa cuneata (Moreau). J Fish Dis 32(7):603–610
López JR, Piñeiro-Vidal M, García-Lamas N, De La Herran R, Navas JI, Hachero-Cruzado I, Santos Y (2010) First isolation of Tenacibaculum soleae from diseased cultured wedge sole, Dicologoglossa cuneata (Moreau), and brill, Scophthalmus rhombus (L.). J Fish Dis 33(3):273–278
López JR, Hamman-Khalifa AM, Navas JI, De la Herran R (2011) Characterization of ISR region and development of a PCR assay for rapid detection of the fish pathogen Tenacibaculum soleae. FEMS Microbiol Lett 324(2):181–188
López JR, Navas JI, Thanantong N, de la Herran R, Sparagano OAE (2012) Simultaneous identification of five marine fish pathogens belonging to the genera Tenacibaculum, Vibrio, Photobacterium and Pseudomonas by reverse line blot hybridization. Aquaculture 324-325:33–38
López-Cortés XA, Nachtigall FM, Olate VR, Araya M, Oyanedel S, Diaz V, Jakob E, Ríos-Momberg M, Santos LS (2017) Fast detection of pathogens in salmon farming industry. Aquaculture 470:17–24
Lujan KM, Eisen JA, Coil DA (2016) Draft genome sequence of Tenacibaculum soleae UCD-KL19. Genome Announc 4(5):e01120–e01116. https://doi.org/10.1128/genomeA.01120-16
Magariños B, Osorio CR, Toranzo AE, Romalde JL (1997) Applicability of ribotyping for intraspecific classification and epidemiological studies of Photobacterium damsela subsp. piscicida. Syst Appl Microbiol 20(4):634–639
Masumura K, Wakabayashi H (1977) An outbreak of gliding bacterial disease in hatchery-born red seabream (Pagrus major) and gilthead (Acanthopagrus schlegeli) fry in Hiroshima. Fish Pathol 12(3):171–177
Mata M, Skarmeta A, Santos Y (2002) A proposed serotyping system for Flavobacterium psychrophilum. Lett Appl Microbiol 35(2):166–170
McVicar AH, White PG (1979) Fin and skin necrosis of cultivated Dover sole Solea solea (L). J Fish Dis 2(6):557–562
McVicar AH, White PG (1982) The prevention and cure of an infectious disease in cultivated juvenile Dover sole, Solea solea (L.). Aquaculture 26(3–4):213–222
National Committee for Clinical Laboratory Standards (2003) Methods for antimicrobial disk susceptibility testing of bacteria isolated from aquatic animals; a report. NCCLS document M42-R. National Committee for Clinical Laboratory Standards, Wayne, PA
Olsen AB, Gulla S, Steinum T, Colquhoun DJ, Nilsen HK, Duchaud E (2017) Multilocus sequence analysis reveals extensive genetic variety within Tenacibaculum spp. associated with ulcers in sea-farmed fish in Norway. Vet Microbiol 205:39–45
Ostland VE, LaTrace C, Morrison D, Ferguson HW (1999) Flexibacter maritimus associated with a bacterial stomatitis in Atlantic salmon smolts reared in net-pens in British Columbia. J Aquat Anim Health 11(1):35–44
Pazos F (1997) Flexibacter maritimus: estudio fenotípico, inmunológico y molecular. Tesis doctoral, Universidad Santiago de Compostela (Santiago de Compostela)
Pazos F, Santos Y, Núñez S, Toranzo AE (1993) Increasing occurrence of Flexibacter maritimus in the marine aquaculture of Spain. FHS/AFS Newsl 21:1–2
Pazos F, Santos Y, Macias AR, Núñez S, Toranzo AE (1996) Evaluation of media for the successful culture of Flexibacter maritimus. J Fish Dis 19(2):193–197
Pépin JF, Emery E (1993) Marine Cytophaga-like bacteria (CLB) isolated from diseased reared sea bass (Dicentrarchus labrax L.) from French Mediterranean coast. Bull Eur Assoc Fish Pathol 13(5):165–167
Pérez- Pascual D, Lunazzi A, Magdelenat G, Rouy Z, Roulet A, López-Roques C, Larocque R, Barbeyron T, Gobet A, Michel G, Bernardet JF, Duchaud E (2017) The complete genome sequence of the fish pathogen Tenacibaculum maritimum provides insights into virulence mechanisms. Front Microbiol 16(8):1–11
Pérez-Sancho M, Vela AI, Awad M, Kostrzewa M, Dominguez L, Fernández-Garayzábal JF (2016) Differentiation of Photobacterium damselae subspecies using matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) in fish isolates. Aquaculture 464:159–164
Pérez-Sancho M, Vela AI, Wiklund T, Kostrzewa M, Domínguez L, Fernández-Garayzábal JF (2017) Differentiation of Flavobacterium psychrophilum from Flavobacterium psychrophilum-like species by MALDI-TOF mass spectrometry. Res Vet Sci 115:345–352
Piñeiro Vidal M, Ruiz de Ocenda M, Santos Y (2009) Susceptibilidad antimicrobiana de los patógenos de peces Tenacibaculum discolor, T gallaicum y T soleae Sesión de Sanidad Animal, XII Congreso Nacional de Acuicultura pp 314–315
Piñeiro-Vidal M (2008) Descripción de tres nuevas especies del Género Tenacibaculum causantes de tenacibaculosis: aspectos taxonómicos y patogenicidad. PhD thesis. Universidad de Santiago de Compostela, Santiago de Compostela, Spain
Piñeiro-Vidal M, Riaza A, Santos Y (2006) Serological typing of Tenacibaculum sp. isolated from diseased turbot and sole cultured in Spain. Proceedings of 3rd International Congress on Aquaculture, Fisheries Technology and Environmental Management (AquaMedit). pp 1–10
Piñeiro-Vidal M, Centeno-Sestelo G, Riaza A, Santos Y (2007) Isolation of pathogenic Tenacibaculum maritimum-related organisms from diseased turbot and sole cultured in the northwest of Spain. Bull Eur Assoc Fish Pathol 27(1):29–35
Piñeiro-Vidal M, Riaza A, Santos Y (2008a) Tenacibaculum discolor sp. nov. and Tenacibaculum gallaicum sp. nov., isolated from sole (Solea senegalensis) and turbot (Psetta maxima) culture systems. Int J Syst Evol Microbiol 58(1):21–25
Piñeiro-Vidal M, Carballas CG, Gómez-Barreiro O, Riaza A, Santos Y (2008b) Tenacibaculum soleae sp. nov., isolated from diseased sole (Solea senegalensis Kaup). Int J Syst Evol Microbiol 58(4):881–885
Piñeiro-Vidal M, Pazos F, Santos Y (2008c) Fatty acid analysis as a chemotaxonomic tool for taxonomic and epidemiological characterization of four fish pathogenic Tenacibaculum species. Lett Appl Microbiol 46(5):548–554
Piñeiro-Vidal M, Gijón D, Zarza C, Santos Y (2012) Tenacibaculum dicentrarchi sp. nov., a novel marine bacteria of the family Flavobacteriaceae isolated from European sea bass (Dicentrarchus labrax, L.). Int J Syst Evol Microbiol 62(2):425–429
Powell M, Carson J, Van-Gelderen R (2004) Experimental induction of gill disease in Atlantic salmon Salmo salar smolts with Tenacibaculum maritimum. Dis Aquat Org 61(3):179–185
Rahman T, Suga K, Kanai K, Sugihara Y (2014) Biological and serological characterization of a non-gliding strain of Tenacibaculum maritimum isolated from a diseased puffer fish Takifugu rubripes. Jpn Soc Fish Pathol 49(3):121–129
Rochat T, Fujiwara-Nagata E, Calvez S, Dalsgaard I, Madsen L, Calteau A, Lunazzi A, Nicolas P, Wiklund T, Bernardet JF, Duchaud E (2017) Genomic characterization of Flavobacterium psychrophilum serotypes and development of a multiplex PCR-based serotyping scheme. Front Microbiol 8:1–9
Sakai T, Iida T, Osatomi K, Kanai K (2007) Detection of type 1 fimbrial genes in fish pathogenic and non-pathogenic Edwardsiella tarda strains by PCR. Fish Pathol 42(2):115–117
Salati F, Cubadda C, Viale I, Kusuda R (2005) Immune response of sea bass Dicentrarchus labrax to Tenacibaculum maritimum antigens. Fish Sci 71(3):563–567
Santos Y, Pazos F, Barja JL (1999) Flexibacter maritimus, causal agent of flexibacteriosis in marine fish. In: ICES identification leaflets for diseases and parasites of fish and shellfish N° 55, International Council for the Exploration of the Sea. ICES, Denmark, pp 1–6
Shoemaker CA, Arias CR, Klesius PH, Welker TL (2005) Technique for identifying Flavobacterium columnare using whole-cell fatty acid profiles. J Aquat Anim Health 17(3):267–274
Singhal N, Kumar M, Kanaujia PK, Virdi JS (2015) MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol 6:1–16
Småge SB, Brevik ØJ, Duesund H, Ottem KF, Watanabe K, Nylund A (2016) Tenacibaculum finnmarkense sp. nov., a fish pathogenic bacterium of the family Flavobacteriaceae isolated from Atlantic salmon. Antonie Van Leeuwenhoek 109(2):273–285
Småge SB, Brevik ØJ, Frisch K, Watanabe K, Duesund H, Nylund A (2017) Concurrent jellyfish blooms and tenacibaculosis outbreaks in northern Norwegian Atlantic salmon (Salmo salar) farms. PLoS One 12(11):e0187476
Småge SB, Frisch K, Vold V, Duesund H, Brevik ØJ, Olsen RH, Sjaatil ST, Klevan A, Brudeseth B, Watanabe K, Nylund A (2018) Induction of tenacibaculosis in Atlantic salmon smolts using Tenacibaculum finnmankense and the evaluation of a whole cell inactivated vaccine. Aquaculture 495:858–864
Soltani M, Burke CM (1994) Responses of fish-pathogenic Cytophaga/Flexibacter-like bacteria (CFLB) to environmental conditions. Bull Eur Assoc Fish Pathol 14(6):185–187
Soltani M, Munday BL, Burke CM (1996) The relative susceptibility of fish to infections by Flexibacter columnaris and Flexibacter maritimus. Aquaculture 140(3):259–264
Suzuki M, Nakagawa Y, Harayama S, Yamamoto S (2001) Phylogenetic analysis and taxonomic study of marine Cytophaga-like bacteria: proposal for Tenacibaculum gen. nov. with Tenacibaculum maritimum comb. nov. and Tenacibaculum ovolyticum comb. nov., and description of Tenacibaculum mesophilum sp. nov. and Tenacibaculum amylolyticum sp. nov. Int J Syst Evol Microbiol 51:1639–1652
Teramoto M, Zhai Z, Komatsu A, Shibayama K, Suzuki M (2016) Genome sequence of the psychrophilic bacterium Tenacibaculum ovolyticum strain da5A-8 isolated from deep seawater. Genome Announc 4(3):e00644–e00616
Toranzo AE, Magariños B, Romalde JL (2005) A review of the main bacterial fish diseases in mariculture systems. Aquaculture 246(1–4):37–61
Torres-Corral Y, Fernández-Álvarez C, Santos Y (2019) Proteomic and molecular fingerprinting for identification and tracking of fish pathogenic Streptococcus. Aquaculture 498:322–334
Toyama T, Kita-Tsukamoto K, Wakabayashi H (1996) Identification of Flexibacter maritimus, Flavobacterium branchiophilum and Cytophaga columnaris by PCR targeted 16S ribosomal DNA. Fish Pathol 31(1):25–31
Tu C, Suga K, Kanai K (2015) A multiplex PCR assay for differentiation of Streptococcus parauberis serotypes. Fish Pathol 50(4):213–215
Van Gelderen R, Carson J, Gudkovs N, Nowak B (2010) Physical characterisation of Tenacibaculum maritimum for vaccine development. J Appl Microbiol 109(5):1668–1676
Vold V (2014) Challenge experiment with field isolates of Tenacibaculum spp. isolated from moribound Atlantic salmon (Salmo salar L). Bergen: Master thesis. University of Bergen
Wakabayashi H, Hikida M, Masumura K (1984) Flexibacter infection in cultured marine fish in Japan. Helgoländer Meeresun 37(1–4):587–593
Wakabayashi H, Hikida M, Masumura K (1986) Flexibacter maritimus sp. nov., a pathogen of marine fishes. Int J Syst Bacteriol 36:396–398
Warsen AE, Krug MJ, LaFrentz S, Stanek DR, Loge FJ, Call DR (2004) Simultaneous discrimination between 15 fish pathogens by using 16S ribosomal DNA PCR and DNA microarrays. Appl Environ Microbiol 70(7):4216–4221
Weisburg WG, Barns S, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703
Welker M (2011) Proteomics for routine identification of microorganisms. Proteomics 11(15):3143–3152
Wilson T, Carson J (2003) Development of sensitive, high throughput one-tube RT-PCR-enzyme hybridization assay to detect selected bacterial fish pathogens. Dis Aquat Org 54:127–134
Wilson T, Carson J, Bowman J (2002) Optimization of one tube PCR-ELISA to detect femtogram amounts of genomic DNA. J Microbiol Methods 51(2):163–170
Wolska K, Szweda P (2012) Genotyping techniques for determining the diversity of microorganisms. In: Caliskan M (ed) Genetic diversity in microorganisms. InTech, Rijeka, pp 53–94
Yardimci R, Timur G (2015) Isolation and identification of Tenacibaculum maritimum, the causative agent of tenacibaculosis in farmed sea bass (Dicentrarchus labrax) on the Aegean Sea coast of Turkey. IJA-Bamidgeh 67:1–10
Yardimci RE, Timur G (2016) Antigenic characterisation of Tenacibaculum maritimum isolates from sea bass (Dicentrarchus labrax, L.) farmed on the Aegean Sea coasts of Turkey. J Aquac Res Dev 7(2):1–4
Acknowledgements
This work was partially funded by the proof of concept “Acelerador de transferencia” 2017 from the Banco de Santander and Universidad de Santiago de Compostela. Clara Fernández-Álvarez is grateful to Banco de Santander and Universidad de Santiago de Compostela for a research contract.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Authors 1 and 2 declare that they have no conflict of interest.
Additional information
Minireview
Submitted to: Applied Microbiology and Biotechnology
Reviewed version of Ms. No. AMAB-D-18-01435
Rights and permissions
About this article
Cite this article
Fernández-Álvarez, C., Santos, Y. Identification and typing of fish pathogenic species of the genus Tenacibaculum. Appl Microbiol Biotechnol 102, 9973–9989 (2018). https://doi.org/10.1007/s00253-018-9370-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9370-1