Abstract
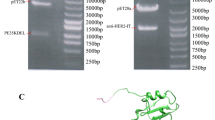
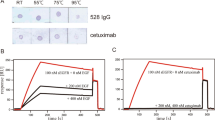
Fragment engineering of monoclonal antibodies (mAbs) has emerged as an excellent paradigm to develop highly efficient therapeutic and/or diagnostic agents. Engineered mAb fragments can be economically produced in bacterial systems using recombinant DNA technologies. In this work, we established recombinant production in Escherichia coli for monovalent antigen-binding fragment (Fab) adopted from a clinically used anticancer mAB drug cetuximab targeting epidermal growth factor receptor (EGFR). Recombinant DNA constructs were designed to express both polypeptide chains comprising Fab in a single vector and to secrete them to bacterial periplasmic space for efficient folding. Particularly, a C-terminal engineering to confer an interchain disulfide bond appeared to be able to enhance its heterodimeric integrity and EGFR-binding activity. Conformational relevance of the purified final product was validated by mass spectrometry and crystal structure at 1.9 Å resolution. Finally, our recombinant cetuximab-Fab was found to have strong binding affinity to EGFR overexpressed in human squamous carcinoma model (A431) cells. Its binding ability was comparable to that of cetuximab. Its EGFR-binding affinity was estimated at approximately 0.7 nM of Kd in vitro, which was quite stronger than the binding affinity of natural ligand EGF. Hence, the results validate that our construction could serve as an efficient platform to produce a recombinant cetuximab-Fab with a retained antigen-binding functionality.





Similar content being viewed by others
References
Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66:213–221
Andersen DC, Reilly DE (2004) Production technologies for monoclonal antibodies and their fragments. Curr Opin Biotechnol 15:456–462
Arbabi-Ghahroudi M, Tanha J, MacKenzie R (2005) Prokaryotic expression of antibodies. Cancer Metastasis Rev 24:501–519
Ayoub D, Jabs W, Resemann A, Evers W, Evans C, Main L, Baessmann C, Wagner-Rousset E, Suckau D, Beck A (2013) Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques. MAbs 5:699–710
Baldassano R, Braegger CP, Escher JC, DeWoody K, Hendricks DF, Keenan GF, Winter HS (2003) Infliximab (REMICADE) therapy in the treatment of pediatric Crohn’s disease. Am J Gastroenterol 98:833–838
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578
Brezski RJ, Jordan RE (2010) Cleavage of IgGs by proteases associated with invasive diseases: an evasion tactic against host immunity? MAbs 2:212–220
Choi JH, Lee SY (2004) Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol 64:625–635
Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TA (2008) Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 358:1109–1117
Cosimi AB, Burton RC, Colvin RB, Goldstein G, Delmonico FL, LaQuaglia MP, Tolkoff-Rubin N, Rubin RH, Herrin JT, Russell PS (1981a) Treatment of acute renal allograft rejection with OKT3 monoclonal antibody. Transplantation 32:535–539
Cosimi AB, Colvin RB, Burton RC, Rubin RH, Goldstein G, Kung PC, Hansen WP, Delmonico FL, Russell PS (1981b) Use of monoclonal antibodies to T-cell subsets for immunologic monitoring and treatment in recipients of renal allografts. N Engl J Med 305:308–314
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Emsley P, Lohkamp B, WG S, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501
Fan Z, Masui H, Altas I, Mendelsohn J (1993) Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of 225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res 53:4322–4328
Gill GN, Kawamoto T, Cochet C, Le A, Sato JD, Masui H, McLeod C, Mendelsohn J (1984) Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem 259:7755–7760
Holliger P, Hudson PJ (2005) Engineered antibody fragments and the rise of single domains. Nat Biotechnol 23:1126–1136
Kawamoto T, Sato JD, Le A, Polikoff J, Sato GH, Mendelsohn J (1983) Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A 80:1337–1341
Kim YP, Park D, Kim JJ, Chi WJ, Lee SH, Lee SY, Kim S, Chung JM, Jeon J, Lee BD, Shin JH, Lee YI, Cho H, Lee JM, Kang HC (2014) Effective therapeutic approach for head and neck cancer by an engineered minibody targeting the EGFR receptor. PLoS One 9:e113442
Kohler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7:301–311
McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK (1998) Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16:2825–2833
Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ (2000) Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 356:385–390
Mendelsohn J (2000) Blockade of receptors for growth factors: an anticancer therapy--the fourth annual Joseph H Burchenal American Association of Cancer Research Clinical Research Award Lecture. Clin Cancer Res 6:747–753
Miller PD (2009) Denosumab: anti-RANKL antibody. Curr Osteoporos Rep 7:18–22
Minor W, Otwinowski Z (1997) HKL2000 (Denzo-SMN) software package. Processing of X-ray diffraction data collected in oscillation mode. Methods in enzymology, macromolecular crystallography. Academic Press, New York
Movva NR, Nakamura K, Inouye M (1980) Amino acid sequence of the signal peptide of ompA protein, a major outer membrane protein of Escherichia coli. J Biol Chem 255:27–29
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF chimera--a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC (2005) Monoclonal antibody successes in the clinic. Nat Biotechnol 23:1073–1078
Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH (1983) Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med 1:511–529
Sockolosky JT, Szoka FC (2013) Periplasmic production via the pET expression system of soluble, bioactive human growth hormone. Protein Expr Purif 87:129–135
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Wlad H, Ballagi A, Bouakaz L, Gu Z, Janson JC (2001) Rapid two-step purification of a recombinant mouse fab fragment expressed in Escherichia coli. Protein Expr Purif 22:325–329
Yokota T, Milenic DE, Whitlow M, Schlom J (1992) Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res 52:3402–3408
Acknowledgments
This work made use of the SEC-MALS and MALDI-TOF machines at Korea Basic Science Institute (KBSI; Ochang, Korea). We thank research staffs at KBSI for their assistance to our operations.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by a grant (C0333276) of Business for Cooperative R&D Program between Industry, Academy, and Research Institute funded by Korea Small and Medium Business Administration in 2015.
Competing interests
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Ji-Hun Kim, Dae-Won Sim, and Dongsun Park contributed equally to this work.
Electronic supplementary material
ESM 1
(PDF 481 kb)
Rights and permissions
About this article
Cite this article
Kim, JH., Sim, DW., Park, D. et al. Bacterial production and structure-functional validation of a recombinant antigen-binding fragment (Fab) of an anti-cancer therapeutic antibody targeting epidermal growth factor receptor. Appl Microbiol Biotechnol 100, 10521–10529 (2016). https://doi.org/10.1007/s00253-016-7717-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7717-z