Abstract
The polyene macrolide group includes important antifungal drugs, to which resistance does not arise readily. Chemical and biological methods have been used in attempts to make polyene antibiotics with fewer toxic side effects. Genome sequencing of producer organisms is contributing to this endeavour, by providing access to new compounds and by enabling yield improvement for polyene analogues obtained by engineered biosynthesis. This recent work is also enhancing bioinformatic methods for deducing the structures of cryptic natural products from their biosynthetic enzymes. The stereostructure of candicidin D has recently been determined by NMR spectroscopy. Genes for the corresponding polyketide synthase have been uncovered in several different genomes. Analysis of this new information strengthens the view that protein sequence motifs can be used to predict double bond geometry in many polyketides.
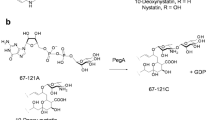
Chemical studies have shown that improved polyenes can be obtained by modifying the mycosamine sugar that is common to most of these compounds. Glycoengineered analogues might be produced by biosynthetic methods, but polyene glycosyltransferases show little tolerance for donors other than GDP-α-D-mycosamine. Genome sequencing has revealed extending glycosyltransferases that add a second sugar to the mycosamine of some polyenes. NppY of Pseudonocardia autotrophica uses UDP-N-acetyl-α-D-glucosamine as donor whereas PegA from Actinoplanes caeruleus uses GDP-α-D-mannose. These two enzymes show 51 % sequence identity and are also closely related to mycosaminyltransferases. These findings will assist attempts to construct glycosyltransferases that transfer alternative UDP- or (d)TDP-linked sugars to polyene macrolactones.








Similar content being viewed by others
References
Abu-Salah (1996) Amphotericin B, an update. Br J Biomed Sci 52:122–133
Alhamadsheh MM, Palaniappan N, DasChoudri S, Reynolds KA (2007) Modular polyketide synthases and cis double bond formation: establishment of activated cis-3-cyclohexylpropenoic acid as the diketide intermediate in phoslactomycin biosynthesis. J Am Chem Soc 129:1910–1911. doi:10.1021/ja068818t
Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, Nieuwkoop AJ, Comellas G, Maryum N, Wang S, Uno BE, Wildeman EL, Gonen T, Rienstra CM, Burke MD (2014) Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol 10:400–406. doi:10.1038/nchembio.1496
Annaval T, Paris C, Leadlay PF, Jacob C, Weissman KJ (2015) Evaluating ketoreductase exchanges as a means of rationally altering polyketide stereochemistry. Chembiochem 16(9):1357–1364. doi:10.1002/cbic.201500113
Aparicio JF, Barreales EG, Payero TD, Vicente CM, de Pedro A, Santos-Aberturas J (2016) Biotechnological production and application of the antibiotic pimaricin: biosynthesis and its regulation. Appl Microbiol Biotechnol 100:61–78. doi:10.1007/s00253-015-7077-0
Bailey CB, Pasman ME, Keatinge-Clay AT (2015) Substrate structure–activity relationships guide rational engineering of modular polyketide synthase ketoreductases. Chem Comm 52:792–795. doi:10.1039/C5CC07315D
Barke J, Seipke RF, Grüschow S, Heavens D, Drou N, Bibb MJ, Goss RJM, Yu DW, Hutchings MI (2010) A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. BMC Biol 8:109–118. doi:10.1186/1741-7007-8-109
Bonnett SA, Whicher JR, Papireddy K, Florova G, Smith JL, Reynolds KA (2013) Structural and stereochemical analysis of a modular polyketide synthase ketoreductase domain required for the generation of a cis-alkene. Chem Biol 20(6):772–783. doi:10.1016/j.chembiol.2013.04.014
Borjihan H, Ogita A, Fujita K-I, Hirasawa E, Tanaka T (2009) The vacuole-targeting fungicidal activity of amphotericin B against the pathogenic fungus Candida albicans and its enhancement by allicin. J Antibiot (Tokyo) 62:691–697. doi:10.1038/ja.2009.103
Brautaset T, Sekurova ON, Sletta H, Ellingsen TE, Strom AR, Valla S, Zotchev SB (2000) Biosynthesis of the polyene antifungal antibiotic nystatin in Streptomyces noursei ATCC 11455: analysis of the gene cluster and deduction of the biosynthetic pathway. Chem Biol 7:395–403. doi:10.1016/S1074-5521(00)00120-4
Brautaset T, Sletta H, Nedal A, Borgos SEF, Degnes KF, Bakke I, Volokhan O, Sekurova ON, Treshalin ID, Mirchink EP, Dikiy A, Ellingsen TE, Zotchev S (2008) Improved antifungal polyene macrolides via engineering of the nystatin biosynthetic genes in Streptomyces noursei. Chem Biol 15(11):1198–1206. doi:10.1016/j.chembiol.2008.08.009
Bruheim P, Borgos SEF, Tsan P, Sletta H, Ellingsen TE, Lancelin JM, Zotchev SB (2004) Chemical diversity of polyene macrolides produced by Streptomyces noursei ATCC 11455 and recombinant strain ERD44 with genetically altered polyketide synthase NysC. Antimicrob Agents Chemother 48:4120–4129. doi:10.1128/AAC.48.11.4120-4129.2004
Caffrey P (2003) Conserved amino acid residues correlating with ketoreductase stereospecificity in modular polyketide synthases. Chembiochem 4:649–662. doi:10.1002/cbic.200300581
Caffrey P, Aparicio JF, Malpartida F, Zotchev SB (2008) Biosynthetic engineering of polyene macrolides towards generation of improved antifungal and antiparasitic agents. Curr Top Med Chem 8:639–653. doi:10.2174/156802608784221479
Cao B, Yao F, Zheng X, Cui D, Shao Y, Zhu C, Deng Z, You D (2012) Genome mining of the biosynthetic gene cluster of the polyene macrolide antibiotic tetramycin and characterization of a P450 monooxygenase involved in the hydroxylation of the tetramycin B polyol segment. Chembiochem 13:2234–2242. doi:10.1002/cbic.201200402
Carmody M, Murphy B, Byrne B, Power P, Rai D, Rawlings B, Caffrey P (2005) Biosynthesis of amphotericin derivatives lacking exocyclic carboxyl groups. J Biol Chem 280:34420–34426. doi:10.1074/jbc.M506689200
Cereghetti D, Carreira E (2006) Amphotericin B: 50 years of chemistry and biochemistry. Synthesis 6:914–942. doi:10.1055/s-2006-926368
Che Q, Li T, Liu X, Yao T, Li J, Gu Q, Li D, Li W, Zhu T (2015) Genome scanning inspired isolation of reedsmycins A–F, polyene-polyol macrolides from Streptomyces sp. CHQ-64. RSC Adv 5:22777–22782. doi:10.1039/C4RA15415K
Chen S, Mao X, Shen Y, Zhou Y, Li J, Wang L, Tao X, Yang L, Wang Y, Zhou X, Deng Z, Wei D (2009) Tailoring the P450 monooxygenase gene for FR-008/candicidin biosynthesis. Appl Environ Microbiol 75(6):1778–1781. doi:10.1128/AEM.00859-08
Cioffi AG, Hou J, Grillo AS, Diaz KA, Burke MD (2015) Restored physiology in protein-deficient yeast by a small molecule channel. J Am Chem Soc 137:10096–10099. doi:10.1021/jacs.5b05765
Corral MJ, Serrano DR, Moreno I, Torrado JJ, Domınguez M, Alunda JM (2014a) Efficacy of low doses of amphotericin B plus allicin against experimental visceral leishmaniasis. J Antimicrob Chemother 69:3268–3274. doi:10.1093/jac/dku290
Corral MJ, González-Sánchez E, Cuquerella M, Alunda JM (2014b) In vitro synergistic effect of amphotericin B and allicin on Leishmania donovani and L. infantum. Antimicrob Agents Chemother 58(3):1596–1602. doi:10.1128/AAC.00710-13
Croatt MP, Carreira EM (2011) Probing the role of the mycosamine C2’-OH on the activity of amphotericin B. Org Lett 13:1390–1393. doi:10.1021/ol2000765
Cui H, Ni X, Shao W, Su J, Su J, Ren J, Xia H (2015) Functional manipulations of the tetramycin positive regulatory gene ttmRIV to enhance the production of tetramycin A and nystatin A1 in Streptomyces ahygroscopicus. J Ind Microbiol Biotechnol 42(9):1273–1282. doi:10.1007/s10295-015-1660-3
Cybulska B, Gadomska I, Mazerski J, Grzybowska J, Borowski E, Cheron M, Bolard J (2000) N-Methyl-N-D-fructosyl amphotericin B methyl ester (MF-AME), a novel antifungal agent of low toxicity: Monomer/micelle control over selective toxicity. Acta Biochim Pol 47:121–131
Davis SA, Vincent BM, Endo MM, Whitesell L, Marchillo K, Andes DR, Lindquist S, Burke MD (2015) Nontoxic antimicrobials that evade drug resistance. Nat Chem Biol 11:481–487. doi:10.1021/jacs.5b05766
De Poire E, Stephens N, Rawlings BJ, Caffrey P (2013) Engineered biosynthesis of disaccharide-modified polyene macrolides. Appl Environ Microbiol 79(19):6156–6159. doi:10.1128/AEM.02197-13
Delattin N, Bruno PA, Cammue BPA, Thevissen K (2014) Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med Chem 6(1):77–90. doi:10.4155/fmc.13.189
Escudero L, Al-Refai A, Nieto C, Laatsch H, Malpartida F, Seco EM (2015) New rimocidin/CE-108 derivatives obtained by a crotonyl-CoA carboxylase/reductase gene disruption in Streptomyces diastaticus var. 108: substrates for the polyene carboxamide synthase PcsA. PLoS One 10(8):e0135891. doi:10.1371/journal.pone.0135891
Essig S, Schmalzbauer B, Bretzke S, Scherer O, Koeberle A, Werz O, Müller R, Menche D (2016) Predictive bioinformatic assignment of methyl-bearing stereocenters: total synthesis and an additional molecular target of ajudazol B. J Org Chem 81(4):1333–1357. doi:10.1021/acs.joc.5b02844
Gao H, Grüschow S, Barke J, Seipke RF, Hill LM, Orivel J, Yu DW, Hutchings M, Goss RJM (2014) Filipins: the first antifungal “weed killers” identified from bacteria isolated from the trap-ant. RSC Adv 4:57267–57270. doi:10.1039/C4RA09875G
Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD (2012) Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A 109:2234–2239. doi:10.1073/pnas.1117280109
Haeder S, Wirth R, Herz H, Spiteller D (2009) Candicidin-producing Streptomyces support leaf-cutting ants to protect their fungus garden against the pathogenic fungus Escovopsis. Proc Natl Acad Sci U S A 106:4742–4746. doi:10.1073/pnas.0812082106
Hartsel S, Bolard J (1996) Amphotericin B: new life for an old drug. Trends Pharm Sci 17:445–449. doi:10.1016/S0165-6147(96)01012-7
Hartsel SC, Weiland TR (2003) Amphotericin B binds to amyloid fibrils and delays their formation: a therapeutic mechanism? Biochemistry 42(20):6228–6233. doi:10.1021/bi0270384
Hutchinson E, Murphy B, Dunne T, Breen C, Rawlings B, Caffrey P (2010) Redesign of polyene macrolide glycosylation: engineered biosynthesis of 19-(O)-perosaminyl-amphoteronolide B. Chem Biol 17:174–182. doi:10.1016/j.chembiol.2010.01.007
Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Mura S (2003) Complete genome sequence and comparative analysis of of the industrial micro-organism Streptomyces avermitilis. Nat Biotechnol 21:526–531. doi:10.1038/nbt820
Janout V, Schell WA, Thevenin D, Yu Y, Perfect JR (2015) Taming amphotericin B. Bioconj Chem 26:2021–2014. doi:10.1021/acs.bioconjchem.5b00463
Jeon BJ, Kim JD, Han JW, Kim BS (2016) Antifungal activity of rimocidin and a new rimocidin derivative BU16 produced by Streptomyces mauvecolor BU16 and their effects on pepper anthracnose. J Appl Microbiol. doi:10.1111/jam.13071
Jiang H, Wang Y-Y, Ran X-X, Fan W-M, Jiang X-H, Guan W-J, Li Y-Q (2013) Improvement of natamycin production by engineering of phosphopantetheinyl transferases in Streptomyces chattanoogensis L10. Appl Environ Microbiol 79(11):3346–3354. doi:10.1128/AEM.00099-13
Jørgensen H, Fjærvik E, Hakvåg S, Bruheim P, Bredholt H, Klinkenberg G, Ellingsen TE, Zotchev SB (2009a) Candicidin biosynthesis gene cluster is widely distributed among Streptomyces spp. isolated from the sediments and the neuston layer of the Trondheim fjord, Norway. Appl Environ Microbiol 75(10):3296–3303. doi:10.1128/AEM.02730-08
Jørgensen H, Degnes KF, Sletta H, Fjaervik E, Dikiy A, Herfindal L, Bruheim P, Klinkenberg G, Bredholt H, Nygård G, Døskeland SO, Ellingsen TE, Zotchev SB (2009b) Biosynthesis of macrolactam BE-14106 involves two distinct PKS systems and amino acid processing enzymes for generation of the aminoacyl starter unit. Chem Biol 16:1109–1121. doi:10.1016/j.chembiol.2009.09.014
Jose PA, Jebakumar SRD (2013) Non-streptomycete actinomycetes nourish the current microbial antibiotic drug discovery. Front Microbiol 4:240. doi:10.3389/fmicb.2013.00240
Keatinge-Clay AT (2007) A tylosin ketoreductase reveals how chirality is determined in polyketides. Chem Biol 14:898–908. doi:10.1016/j.chembiol.2007.07.009
Keatinge-Clay AT (2016) Stereocontrol within polyketide assembly lines. Nat Prod Rep. doi:10.1039/c5np00092k
Kells PM, Ouellet H, Santos-Aberturas J, Aparicio JF, Podust LM (2010) Structure of cytochrome P450 PimD suggests epoxidation of the polyene macrolide pimaricin occurs via a hydroperoxoferric intermediate. Chem Biol 17(8):841–851. doi:10.1016/j.chembiol.2010.05.026
Kim BG, Lee MJ, Seo JY, Hwang YB, Lee MY, Han K, Sherman DH, Kim ES (2009) Identification of functionally clustered nystatin-like biosynthetic genes in a rare actinomycetes, Pseudonocardia autotrophica. J Ind Microbiol Biotechnol 36:1425–1434. doi:10.1007/s10295-009-0629-5
Kim D-G, Moon K, Kim S-H, Park S-H, Park S, Lee SK, Oh K-B, Shin J, Oh D-C (2012) Bahamaolides A and B, antifungal polyene polyol macrolides from the marine actinomycete Streptomyces sp. J Nat Prod 75:959–967. doi:10.1021/np3001915
Kim HJ, Kim MK, Lee MJ, Won HJ, Choi SS, Kim ES (2015) Post-PKS Tailoring steps of a disaccharide-containing polyene NPP in Pseudonocardia autotrophica. PLoS one 10(4):e0123270. doi:10.1371/journal.pone.0123270
King JD, Poon KKH, Webb NA, Anderson EM, McNally DJ, Brisson J-R, Messner P, Garavito RM, Lam JS (2009) The structural basis for catalytic function of GMD and RMD, two closely related enzymes from the GDP-d-rhamnose biosynthesis pathway. FEBS J 276:2686–2700. doi:10.1111/j.1742-4658.2009.06993.x
Komaki H, Izumikawa M, J-y U, Nakashima T, Khan ST, Takagi M, Shin-ya K (2009) Discovery of a pimaricin analogue JBIR-13, from Streptomyces bicolor NBRC 12746 as predicted by sequence analysis of type I polyketide synthase gene. Appl Microbiol Biotechnol 83:127–133. doi:10.1007/s00253-008-1849-8
Komaki H, Ichikawa N, Hosoyama A, Fujita N, Igarashi Y (2015) Draft genome sequence of marine-derived Streptomyces sp. TP-A0873, a producer of a pyrrolizidine alkaloid bohemamine. Genome Announc 3(1):e00008–e00015. doi:10.1128/genomeA.00008-15
Kong D, Lee M-J, Lin S, Kim E-S (2013) Biosynthesis and pathway engineering of antifungal polyene macrolides in actinomycetes. J Ind Microbiol Biotechnol 40:529–543. doi:10.1007/s10295-013-1258-6
Kwan DH, Sun Y, Schulz F, Hong H, Popovic B, Sim-Stark JC, Haydock SF, Leadlay PF (2008) Prediction and manipulation of the stereochemistry of enoylreduction in modular polyketide synthases. Chem Biol 15:1231–1240. doi:10.1016/j.chembiol.2008.09.012
Kwon HK, Kauffman CA, Jensen PR, Fenical W (2009) Marinisporolides, polyene polyol macrolides from a marine actinomycete of the new genus Marinispora. J Org Chem 74(2):675–684. doi:10.1021/jo801944d
Labonte JW, Townsend CA (2013) Active site comparisons and catalytic mechanisms of the hot dog superfamily. Chem Rev 113:2182–2204. doi:10.1021/cr300169a
Lee MJ, Kong D, Han KB, Sherman DH, Bai L, Deng Z, Lin S, Kim ES (2012) Structural analysis and biosynthetic engineering of a solubility-improved and less-hemolytic nystatin-like polyene in Pseudonocardia autotrophica. Appl Microbiol Biotechnol 95:157–168. doi:10.1007/s00253-012-3955-x
Lei X, Kong L, Zhang C, Liu Q, Yao F, Zhang W, Zixin Deng Z, You D (2013) In vivo investigation of the substrate recognition capability and activity affecting amino acid residues of glycosyltransferase FscMI in the biosynthesis of candicidin. Mol BioSyst 9:422–430. doi:10.1039/C2MB25464F
Lemke A, Kiderlin AF, Kayser O (2005) Amphotericin B. Appl Microbiol Biotechnol 68:151–162. doi:10.1007/s00253-005-1955-9
Letek M, Mateos LM, Gil JA (2014) Antimicrobial compounds. In: Villa TG, Veiga-Crespo P (eds) Genetic analysis and manipulation as a way to produce more effective antifungal compounds. Springer Verlag, Berlin Heidelberg. doi:10.1007/978-3-642-40444-3_7
Lin T-Y, Chin CR, Everitt AR, Clare S, Perreira JM, Savidis G, Aker AM, John SP, Sarlah D, Carreira EM, Elledge SJ, Kellam P, Brass AL (2013) Amphotericin B increases influenza A virus infection by preventing IFITM3-mediated restriction. Cell Rep 5:895–908. doi:10.1016/j.celrep.2013.10.033
Liu Q, Zhou Y, Liu T, Deng Z (2015a) Poster 75. Society for Industrial Microbiology and Biotechnology meeting. In: A promising chassis derived from Streptomyces sp. FR-008 and its development for polyketide derived natural products heterologous overproduction system. Natural Product Discovery and Development in the Post Genomic Era, San Diego CA Jan 11–14 2015
Liu SP, Yuan PH, Wan YY, Liu XF, Zhou ZX, Bu QT, Yu P, Jiang H, Li YQ (2015b) Generation of the natamycin analogues by gene engineering of natamycin biosynthetic genes in Streptomyces chattanoogensis L10. Microbiol Res 173:25–33. doi:10.1016/j.micres.2015.01.013
López D, Fischbach MA, Chu F, Losick R, Kolter R (2008) Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci U S A 106(1):280–285. doi:10.1073/pnas.0810940106253_7474.docx
Magarvey NA, Haltli B, He M, Greenstein M, Hucul JA (2006) Biosynthetic pathway for mannopeptimycins, lipoglycopeptide antibiotics active against drug-resistant gram-positive pathogens. Antimicrob Agents Chemother 50(6):2167–2177. doi:10.1128/AAC.01545-05
Mendes MV, Recio E, Anton N, Guerra SM, Santos-Aberturas J, Martin JF, Aparicio JF (2007) Cholesterol oxidases act as signalling proteins for the biosynthesis of the polyene macrolide pimaricin. Chem Biol 14:279–290. doi:10.1016/j.chembiol.2007.01.010
Mesa-Arango AC, Scorzoni L, Zaragoza O (2012) It only takes one to do many jobs: amphotericin B as antifungal and immunomodulatory drug. Front Microbiol 3:286. doi:10.3389/fmicb.2012.00286
Murphy B, Anderson K, Borissow C, Caffrey P, Griffith G, Hearn J, Ibrahim O, Khan N, Lamburn N, Lee M, Pugh K, Rawlings B (2010) Isolation and characterisation of amphotericin B analogues and truncated intermediates produced by genetic engineering of Streptomyces nodosus. Org Biomolec Chem 8:3758–3770. doi:10.1039/b922074g
Nedal A, Sletta H, Brautaset T, Borgos SEF, Sekurova ON, Ellingsen TE, Zotchev SB (2007) Analysis of the mycosamine biosynthesis and attachment genes in the nystatin biosynthetic gene cluster of Streptomyces noursei ATCC 11455. Appl Environ Microbiol 73(22):7400–7407. doi:10.1128/AEM.01122-07
Nic Lochlainn L, Caffrey P (2009) Phosphomannose isomerase and phosphomannomutase gene disruptions in Streptomyces nodosus: impact on amphotericin biosynthesis and implications for glycosylation engineering. Metab Eng 11:40–47. doi:10.1016/j.ymben.2008.08.007
Nishimura S, Tokukura M, Ochi J, Yoshida M, Kakeya H (2014) Balance between exocytosis and endocytosis determines the efficacy of sterol-targeting antibiotics. Chem Biol 21(12):1690–1699. doi:10.1016/j.chembiol.2014.10.014
Ogita A, Fujita K-I, Tanaka T (2012) Enhancing effects on vacuole-targeting fungicidal activity of amphotericin B. Front Microbiol 3(100):1–8. doi:10.3389/fmicb.2012.00100
Olano C, Garcia I, González A, Rodriguez A, Rozas D, Rubio J, Sánchez-Hidalgo M, Brana AF, Méndez C, Salas JA (2014) Activation and identification of five clusters for secondary metabolites in Streptomyces albus J1074. Microb Biotechnol 7(3):242–256. doi:10.1111/1751-7915.12116
Paddon CJ, Keasling JD (2014) Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat Rev Microbiol 12:355–367. doi:10.1038/nrmicro3240
Palacios DS, Anderson TM, Burke MD (2007) A post-PKS oxidation of the amphotericin B skeleton predicted to be critical for channel formation is not required for potent antifungal activity. J Am Chem Soc 129:13804–13805. doi:10.1073/pnas.1015023108
Palaniappan N, Alhamadsheh M, Reynolds KA (2008) cis-Δ2,3-Double bond of phosplactomycins is generated by a post-PKS tailoring enzyme. J Am Chem Soc 130:12236–12237. doi:10.1021/ja8044162
Pawlak J, Sowinski P, Borowski E, Gariboldi P (1995) Stereostructure of perimycin A. J Antibiot (Tokyo) 48:1034–1038
Payero TD, Vicente CM, Rumbero A, Barreales EG, Santos-Aberturas J, de Pedro A, Aparicio JF (2015) Functional analysis of filipin tailoring genes from Streptomyces filipinensis reveals alternative routes in filipin III biosynthesis and yields bioactive derivatives. Microb Cell Factories 14:114. doi:10.1186/s12934-015-0307-4
Perlova O, Gerth K, Kaiser O, Hans A, Müller R (2006) Identification and analysis of the chivosazol biosynthetic gene cluster from the myxobacterial model strain Sorangium cellulosum So ce56. J Biotechnol 121(2):174–191. doi:10.1016/j.jbiotec.2005.10.011
Preobrazhenskaya MN, Olsufyeva EN, Tevyashova AN, Printsevskaya SS, Solovieva SE, Reznikova MI, Trenin AS, Galatenko OA, Treshalin ID, Pereverzeva ER, Mirchink EP, Zotchev SB (2010) Synthesis and study of the antifungal activity of new mono- and disubstituted derivatives of a genetically engineered polyene antibiotic 28,29-didehydronystatin A1(S44HP). J Antibiot (Tokyo) 63:55–64. doi:10.1038/ja.2009.118
Qi Z, Kang Q, Jiang C, Han M, Bai L (2015) Engineered biosynthesis of pimaricin derivatives with improved antifungal activity and reduced cytotoxicity. Appl Microbiol Biotechnol 99:6745–6752. doi:10.1007/s00253-015-6635-9
Recio E, Colinas A, Rumbero A, Aparicio JF, Martin JF (2004) PI factor a novel quorum-sensing inducer elecits pimaricin production in Streptomyces natalensis. J Biol Chem 279:41586–41593. doi:10.1074/jbc.M402340200
Reid R, Piagentini M, Rodriguez E, Ashley G, Viswanathan N, Carney J, Santi DV, Hutchinson CR, McDaniel R (2003) A model of structure and catalysis for ketoreductase domains in modular polyketide synthases. Biochemistry 42:72–79. doi:10.1021/bi0268706
Rix U, Fischer C, Remsing LL, Rohr J (2002) Modification of post-PKS tailoring steps through combinatorial biosynthesis. Nat Prod Rep 19:542–580. doi:10.1039/B103920M
Ro D-K, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943. doi:10.1038/nature04640
Roethl E, Gassner M, Krenn BM, Romanovskaya-Romanko EA, Seper H, Romanova J, Nakowitsch S, Sturlan S, Wolschek M, Sirotkin A, Kiselev O, Muster T, Egorov A (2011) Antimycotic-antibiotic amphotericin B promotes influenza virus replication in cell culture. J Virol 85(21):11139–11145. doi:10.1128/JVI.00169-11
Rolón M, Seco EM, Vega C, Nogal JJ, Escario JA, Gómez-Barrio A, Malpartida F (2006) Selective activity of polyene macrolides produced by genetically modified Streptomyces on Trypanosoma cruzi. Int J Antimicrob Agents 28(2):104–109. doi:10.1016/j.ijantimicag.2006.02.025
Santos-Aberturas J, Engel J, Dickerhoff J, Dörr M, Rudroff F, Weisz K, Bornscheuer UT (2015) Exploration of the substrate promiscuity of biosynthetic tailoring enzymes as a new source of structural diversity for polyene macrolide antifungals. ChemCatChem 7(3):490–500. doi:10.1002/cctc.201402773
Schell U, Haydock SF, Kaja AL, Carletti I, Lill RE, Read E, Sheehan LS, Low L, Fernandez M-J, Grolle F, McArthur HAI, Sheridan RM, Leadlay PF, Wilkinson B, Gaisser S (2008) Engineered biosynthesis of hybrid macrolide polyketides containing D-angolosamine and D-mycaminose moieties. Org Biomol Chem 6:3315–3327. doi:10.1039/b807914e
Schulze CJ, Donia MS, Siqueira-Neto JL, Ray D, Raskatov JA, Green RE, McKerrow JH, Fischbach MA, Linington RG (2015) Genome-directed lead discovery: biosynthesis, structure elucidation, and biological evaluation of two families of polyene macrolactams against Trypanosoma brucei. ACS Chem Biol 10(10):2373–2381. doi:10.1021/acschembio.5b00308
Seco EM, Miranzo D, Nieto C, Malpartida F (2010) The pcsA gene from Streptomyces diastaticus var 108 encodes a polyene carboxamide synthase with broad substrate specificity for polyene amide biosynthesis. Appl Microbiol Biotechnol 85:1797–1807. doi:10.1007/s00253-009-2193-3
Seipke RF, Barke J, Brearley C, Hill L, Yu DW, Goss RJ, Hutchings MI (2011a) A single Streptomyces symbiont makes multiple antifungals to support the fungus farming ant Acromyrmex octospinosus. PLoS one 6(8):e22028. doi:10.1371/journal.pone.0022028
Seipke RF, Crossman L, Drou N, Heavens D, Bibb MJ, Caccamo M, Hutchings MI (2011b) Draft genome sequence of Streptomyces strain S4, a symbiont of the leaf-cutting ant Acromyrmex octospinosus. J Bacteriol 193(16):4270–4271. doi:10.1128/JB.05275-11
Seipke RF, Grüschow S, Goss RJM, Hutchings MI (2012) Isolating antifungals from fungus-growing ant symbionts using a genome-guided chemistry approach. Methods Enzymol 517:47–70. doi:10.1016/B978-0-12-404634-4.00003-6
Siskos AP, Baerga-Ortiz A, Bali S, Stein V, Mamdani H, Spiteller D, Popvic B, Spencer JB, Staunton J, Weissman KJ, Leadlay PF (2005) Molecular basis of Celmer’s rules: stereochemistry of catalysis by isolated ketoreductase domains from modular polyketide synthases. Chem Biol 12:1145–1153. doi:10.1016/j.chembiol.2005.08.017
Sletta H, Borgos SEF, Bruheim P, Sekurova ON, Grasdalen H, Aune R, Ellingsen TE, Zotchev SB (2005) Nystatin biosynthesis and transport: nysH and nysG genes encoding a putative ABC transporter system in Streptomyces noursei ATCC 11455 are required for efficient conversion of 10-deoxynystatin to nystatin. Antimicrob Agents Chemother 49:4576–4583. doi:10.1128/AAC.49.11.4576-4583.2005
Smith NW, Annunziata O, Dzyuba SV (2009) Amphotericin B interactions with soluble oligomers of amyloid Aβ1-42 peptide. Bioorg Med Chem 17(6):2366–2370. doi:10.1016/j.bmc.2009.02.016
Sowinski P, Gariboldi P, Czerwinski A, Borowski E (1989a) The structure of vacidin A, an aromatic heptaene macrolide antibiotic. I. Complete assignment of the 1H NMR spectrum and geometry of the polyene chromophore. J Antibiot (Tokyo) 62(9):1631–1638
Sowinski P, Gariboldi P, Pawlak JK, Borowski E (1989b) The structure of vacidin A, an aromatic heptaene macrolide antibiotic. II. Stereochemistry of the antibiotic. J Antibiot (Tokyo) 62(9):1639–1642
Sowinski P, Pawlak J, Borowski E, Gariboldi P (1995) Stereostructure of gedamycin. Pol J Chem 69:213–217
Stephens N, Rawlings B, Caffrey P (2012) Streptomyces nodosus host strains optimized for polyene glycosylation engineering. Biosci Biotechnol Biochem 76:384–387. doi:10.1271/bbb.110673
Stephens N, Rawlings B, Caffrey P (2013) Versatility of enzymes catalyzing late steps in polyene 67-121C biosynthesis. Biosci Biotechnol Biochem 77:880–883. doi:10.1271/bbb.120961
Svahn S, Chryssanthou E, Olsen B, Bohlin L, Göransson U (2015) Penicillium nalgiovense Laxa isolated from Antarctica is a new source of the antifungal metabolite amphotericin B. Fungal Biol Biotechnol 2:1–8. doi:10.1186/s40694-014-0011-x
Sweeney P, Murphy CD, Caffrey P (2016) Exploiting the genome sequence of Streptomyces nodosus for enhanced antibiotic production. Appl Microbiol Biotechnol 100:1285–1295. doi:10.1007/s00253-015-7060-9
Szlinder-Richert J, Mazerski J, Cybulska B, Grzybowska J, Borowski E (2001) MFAME, N-methyl-N-D-fructosyl amphotericin B methyl ester, a new amphotericin B derivative of low toxicity: relationship between self-association and effects on red blood cells. Biochim Biophys Acta 1528(1):15–24. doi:10.1016/S0304-4165(01)00166-0
Szpilman AM, Cereghetti DM, Wurtz NR, Manthorpe JM, Carreira EM (2008) Synthesis of 35-deoxyamphotericin B methyl ester: a strategy for molecular editing. Angew Chemie Int Ed Engl 47:4335–4338. doi:10.1002/anie.200800589
Szwarc K, Szczeblewski P, Sowiński P, Borowski E, Pawlak J (2015a) The stereostructure of candicidin D. J Antibiot (Tokyo) 68(8):504–510. doi:10.1038/ja.2015.17
Szwarc K, Szczeblewski P, Sowiński P, Borowski E, Jan Pawlak J (2015b) The structure, including stereochemistry, of levorin A1. Magn Reson Chem 53:479–484. doi:10.1002/mrc.4229
Tang J, Liu X, Peng J, Tang Y, Zhang Y (2015) Genome sequence and genome mining of a marine-derived antifungal bacterium Streptomyces sp. M10. Appl Microbiol Biotechnol 99:2763–2772. doi:10.1007/s00253-015-6453-0
Tevyashova AN, Olsufyeva EN, Solovieva SE, Printsevskaya SS, Reznikova MI, Trenin AS, Galatenko OA, Treshalin ID, Pereverzeva ER, Mirchink EP, Isakova EB, Zotchev SB, Preobrazhenskaya MN (2013) Structure-antifungal activity relationships of polyene antibiotics of the amphotericin B group. Antimicrob Agents Chemother 57(8):3815–3822. doi:10.1128/AAC.00270-13
Tietz JI, Mitchell DA (2015) Using genomics for natural product structure elucidation. Curr Top Med Chem:16. doi:10.2174/1568026616666151012111439
Valenzano CR, Lawson RJ, Chen AY, Khosla C, Cane DE (2009) The biochemical basis for stereochemical control in polyketide biosynthesis. J Am Chem Soc 131(51):18501–18511. doi:10.1021/ja908296m
Vergnolle O, Hahn F, Baerga-Ortiz A, Leadlay PF, Andexer JN (2011) Stereoselectivity of isolated dehydratase domains of the borrelidin polyketide synthase: implications for cis double bond formation. Chembiochem 12:1011–1014. doi:10.1002/cbic.20110001
Vincent BM, Lancaster AK, Scherz-Shouval R, Whitesell L, Lindquist S (2013) Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol 11(10):e1001692. doi:10.1371/journal.pbio.1001692
Volokhan O, Sletta H, Ellingsen TE, Zotchev SB (2006) Characterization of the P450 monooxygenase NysL, responsible for C-10 hydroxylation during biosynthesis of the polyene macrolide antibiotic nystatin in Streptomyces noursei. Appl Environ Microbiol 72(4):2514-2519. doi:10.1128/AEM.72.4.2514-2519.2006
Weissman KJ, Timoney M, Bycroft M, Grice P, Hanefeld U, Staunton J, Leadlay PF (1997) The molecular basis of Celmer’s rules: the stereochemistry of the condensation step in chain extension on the erythromycin polyketide synthase. Biochemistry 36(45):13849–13855. doi:10.1021/bi971566b
Wilcock BC, Endo MM, Uno BE, Burke MD (2013) C2′-OH of amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. J Am Chem Soc 135:8488–8491. doi:10.1021/ja403255s
Wright JJ, Greeves D, Mallams AK, Picker DH (1977) Structural elucidation of heptaene macrolide antibiotics 67-121A and 67-121C. J Chem Soc Chem Commun 1977:710–712. doi:10.1039/C39770000710
Yamamoto T, Umegawa Y, Tsuchikawa H, Matsumori N, Hanashima S, Murata M, Haser R, Rawlings BJ, Caffrey P (2015) Role of polyol moiety of amphotericin B in ion channel formation and sterol selectivity in bilayer membrane. Bioorg Med Chem 23:5782–5788. doi:10.1016/j.bmc.2015.07.009
Yu J, Li M, Wilkins J, Ding S, Swartz TH, Esposito AM, Zheng Y-M, Freed EO, Liang C, Chen BK, Liu S-L (2015) IFITM proteins restrict HIV-1 infection by antagonizing the envelope glycoprotein. Cell Rep 13:145–156. doi:10.1016/j.celrep.2015.08.055
Zhang C, Moretti R, Jiang J, Thorson JS (2008) The in vitro characterisation of polyene glycosyltransferases AmphDI and NysDI. Chembiochem 9:2506–2514. doi:10.1002/cbic.200800349
Zhang P, Zhao Z, Li H, Chen X-L, Deng Z, Bai L, Pang X (2015) Production of the antibiotic FR008/candicidin in Streptomyces sp. FR008 is co-regulated by two regulators, FscRI and FscRIV, from different transcription factor families. Microbiology 161:539–552. doi:10.1099/mic.0.000033
Zheng J, Piasecki SK, Keatinge-Clay AT (2013) Structural studies of an A2-type modular polyketide synthase ketoreductase reveal features controlling α-substituent stereochemistry. ACS Chem Biol 8(9):1964–1971. doi:10.1021/cb400161g
Zidovetzki R, Levitan I (2007) Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta 1768(6):1311–1324. doi:10.1016/j.bbamem.2007.03.026
Zielinksi J, Jereczek E, Sowlinski P, Falowski L, Rudowski A, Borowski E (1979) The structure of a novel sugar component of polyene macrolide antibiotics: 2, 6 dideoxy-L-ribohexopyranose. J Antibiot (Tokyo) 32(6):565–568
Acknowledgments
Work in the authors’ laboratory has been supported by Science Foundation Ireland, grant number 09/RFP/GEN2132.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors
Rights and permissions
About this article
Cite this article
Caffrey, P., De Poire, E., Sheehan, J. et al. Polyene macrolide biosynthesis in streptomycetes and related bacteria: recent advances from genome sequencing and experimental studies. Appl Microbiol Biotechnol 100, 3893–3908 (2016). https://doi.org/10.1007/s00253-016-7474-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7474-z