Abstract
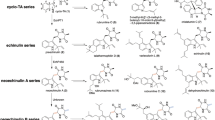
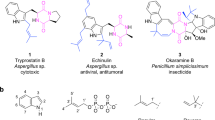
Prenylated tryptophan-containing cyclic dipeptides are found in different fungi and serve as precursors for the biosynthesis of diverse biologically active secondary metabolites. They show distinct and usually higher biological and pharmacological activities than the respective non-prenylated dipeptides. Successful production of such compounds were achieved by a new approach based on the coexpression of ftmPS, a non-ribosomal peptide synthetase from Neosartorya fischeri, with three cyclic dipeptide prenyltransferase genes from different biosynthetic gene clusters in Aspergillus nidulans. The genes are expressed under the control of constitutive gpdA promoter and trpC terminator. Expression of ftmPS alone resulted in the formation of the expected cyclic dipeptide brevianamide F with a yield of up to 36.9 mg l−1. Introducing the reverse C2-prenyltransferase gene cdpC2PT as well as the reverse C3-prenyltransferase gene cdpNPT into a ftmPS mutant yielded reversely C2- and C3-prenylated derivatives, respectively. Coexpression of ftmPS with the reverse C3-prenyltransferase gene cdpC3PT resulted in the formation of N1-regularly, C2-, and C3-reversely prenylated derivatives. The prenyl transfer reactions catalyzed by CdpC2PT, CdpNPT, and CdpC3PT observed in this study correspond well to those detected with purified proteins. The yields of the detected prenylated products were found to be up to 12.2 mg l−1. The results presented in this study show the potential of synthetic biology for production of prenylated compounds.


Similar content being viewed by others
References
Ali H, Ries MI, Nijland JG, Lankhorst PP, Hankemeier T, Bovenberg RA, Vreeken RJ, Driessen AJ (2013) A branched biosynthetic pathway is involved in production of roquefortine and related compounds in Penicillium chrysogenum. PLoS One 8:e65328
Ames BD, Walsh CT (2010) Anthranilate-activating modules from fungal nonribosomal peptide assembly lines. Biochemistry 49:3351–3365
Ballance DJ, Buxton FP, Turner G (1983) Transformation of Aspergillus nidulans by the orotidine-5'-phosphate decarboxylase gene of Neurospora crassa. Biochem Biophys Res Commun 112:284–289
Borthwick AD (2012) 2,5-diketopiperazines: synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem Rev 112:3641–3716
Bouhired S, Weber M, Kempf-Sontag A, Keller NP, Hoffmeister D (2007) Accurate prediction of the Aspergillus nidulans terrequinone gene cluster boundaries using the transcriptional regulator LaeA. Fungal Genet Biol 44:1134–1145
Bradshaw RE (2006) From protoplast to gene clusters. Mycologist 20:133–139
Brakhage AA (2013) Regulation of fungal secondary metabolism. Nat Rev Microbiol 11:21–32
Brakhage AA, Schroeckh V (2011) Fungal secondary metabolites—strategies to activate silent gene clusters. Fungal Genet Biol 48:15–22
Caballero E, Avendañno C, Menéndez JC (2003) Brief total synthesis of the cell cycle inhibitor tryprostatin B and related preparation of its alanine analogue. J Org Chem 68:6944–6951
Calvo AM, Bok J, Brooks W, Keller NP (2004) veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl Environ Microbiol 70:4733–4739
Cramer RA Jr, Stajich JE, Yamanaka Y, Dietrich FS, Steinbach WJ, Perfect JR (2006) Phylogenomic analysis of non-ribosomal peptide synthetases in the genus Aspergillus. Gene 383:24–32
Cui CB, Kakeya H, Okada G, Onose R, Osada H (1996) Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. I. taxonomy, fermentation, isolation and biological properties. J Antibiot 49:527–533
Ding Y, Wet JR, Cavalcoli J, Li S, Greshock TJ, Miller KA, Finefield JM, Sunderhaus JD, McAfoos TJ, Tsukamoto S, Williams RM, Sherman DH (2010) Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp. J Am Chem Soc 132:12733–12740
Frisvad JC, Rank C, Nielsen KF, Larsen TO (2009) Metabolomics of Aspergillus fumigatus. Med Mycol 47:S53–S71
García-Estrada C, Ullán RV, Álbillos SM, Fernández-Bodega MA, Durek P, von DH, Martín JF (2011) A single cluster of coregulated genes encodes the biosynthesis of the mycotoxins roquefortine C and meleagrin in Penicillium chrysogenum. Chem Biol 18:1499–1512
Grundmann A, Li S-M (2005) Overproduction, purification and characterization of FtmPT1, a brevianamide F prenyltransferase from Aspergillus fumigatus. Microbiology 151:2199–2207
Gu B, He S, Yan X, Zhang L (2013) Tentative biosynthetic pathways of some microbial diketopiperazines. Appl Microbiol Biotechnol 97:8439–8453
Li S-M (2010) Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep 27:57–78
Li S-M (2011) Genome mining and biosynthesis of fumitremorgin-type alkaloids in ascomycetes. J Antibiot 64:45–49
Maiya S, Grundmann A, Li S-M, Turner G (2006) The fumitremorgin gene cluster of Aspergillus fumigatus: identification of a gene encoding brevianamide F synthetase. Chembiochem 7:1062–1069
Maiya S, Grundmann A, Li S-M, Turner G (2009) Improved tryprostatin B production by heterologous gene expression in Aspergillus nidulans. Fungal Genet Biol 46:436–440
Marahiel MA (1992) Multidomain enzymes involved in peptide synthesis. FEBS Lett 307:40–43
Minto RE, Townsend CA (1997) Enzymology and molecular biology of aflatoxin biosynthesis. Chem Rev 97:2537–2556
Mundt K, Li S-M (2013) CdpC2PT, a reverse prenyltransferase from Neosartorya fischeri with distinct substrate preference from known C2-prenyltransferases. Microbiology 159:2169–2179
Mundt K, Wollinsky B, Ruan HL, Zhu T, Li S-M (2012) Identification of the verruculogen prenyltransferase FtmPT3 by a combination of chemical, bioinformatic and biochemical approaches. Chembiochem 13:2583–2592
Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, Oakley BR (2006) A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172:1557–1566
Punt PJ, Zegers ND, Busscher M, Pouwels PH, van den Hondel CA (1991) Intracellular and extracellular production of proteins in Aspergillus under the control of expression signals of the highly expressed Aspergillus nidulans gpdA gene. J Biotechnol 17:19–33
Röttig M, Medema MH, Blin K, Weber T, Rausch C, Kohlbacher O (2011) NRPSpredictor2—a web server for predicting NRPS adenylation domain specificity. Nucleic Acids Res 39:W362–W367
Sakai K, Kinoshita H, Nihira T (2012) Heterologous expression system in Aspergillus oryzae for fungal biosynthetic gene clusters of secondary metabolites. Appl Microbiol Biotechnol 93:2011–2022
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schuller JM, Zocher G, Liebhold M, Xie X, Stahl M, Li S-M, Stehle T (2012) Structure and catalytic mechanism of a cyclic dipeptide prenyltransferase with broad substrate promiscuity. J Mol Biol 422:87–99
Shimizu K, Keller NP (2001) Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:600
Tilburn J, Scazzocchio C, Taylor GG, Zabicky-Zissman JH, Lockington RA, Davies RW (1983) Transformation by integration in Aspergillus nidulans. Gene 26:205–221
Usui T, Kondoh M, Cui CB, Mayumi T, Osada H (1998) Tryprostatin A, a specific and novel inhibitor of microtubule assembly. Biochem J 333:543–548
van Loevezijn A, Allen JD, Schinkel AH, Koomen GJ (2001) Inhibition of BCRP-mediated drug efflux by fumitremorgin-type indolyl diketopiperazines. Bioorg Med Chem Lett 11:29–32
Weber T, Marahiel MA (2001) Exploring the domain structure of modular nonribosomal peptide synthetases. Structure 9:R3–R9
Williams RM, Stocking EM, Sanz-Cervera JF (2000) Biosynthesis of prenylated alkaloids derived from tryptophan. Top Curr Chem 209:97–173
Wollinsky B, Ludwig L, Hamacher A, Yu X, Kassack MU, Li S-M (2012) Prenylation at the indole ring leads to a significant increase of cytotoxicity of tryptophan-containing cyclic dipeptides. Bioorg Med Chem Lett 22:3866–3869
Yelton MM, Hamer JE, Timberlake WE (1984) Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A 81:1470–1474
Yin W-B, Ruan H-L, Westrich L, Grundmann A, Li S-M (2007) CdpNPT, an N-prenyltransferase from Aspergillus fumigatus: overproduction, purification and biochemical characterisation. Chembiochem 8:1154–1161
Yin W-B, Xie X-L, Matuschek M, Li S-M (2010a) Reconstruction of pyrrolo[2,3-b]indoles carrying an a-configured reverse C3-dimethylallyl moiety by using recombinant enzymes. Org Biomol Chem 8:1133–1141
Yin W-B, Yu X, Xie X-L, Li S-M (2010b) Preparation of pyrrolo[2,3-b]indoles carrying a ß-configured reverse C3-dimethylallyl moiety by using a recombinant prenyltransferase CdpC3PT. Org Biomol Chem 8:2430–2438
Yu X, Li S-M (2012) Prenyltransferases of the dimethylallyltryptophan synthase superfamily. Methods Enzymol 516:259–278
Yu X, Zocher G, Xie X, Liebhold M, Schütz S, Stehle T, Li S-M (2013) Catalytic mechanism of stereospecific formation of cis-configured prenylated pyrroloindoline diketopiperazines by indole prenyltransferases. Chem Biol 20:1492–1501
Zheng L, Baumann U, Reymond JL (2004) An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res 32:e115
Acknowledgments
This project and Dr. K. Mundt are financially supported by the LOEWE program of the State of Hessen (SynMikro to S.-M. Li). We thank Dr. G. Laufenberg and Dr. R. Ortmann for taking mass and NMR spectra, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Carsten Wunsch and Kathrin Mundt contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1084 kb)
Rights and permissions
About this article
Cite this article
Wunsch, C., Mundt, K. & Li, SM. Targeted production of secondary metabolites by coexpression of non-ribosomal peptide synthetase and prenyltransferase genes in Aspergillus . Appl Microbiol Biotechnol 99, 4213–4223 (2015). https://doi.org/10.1007/s00253-015-6490-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6490-8