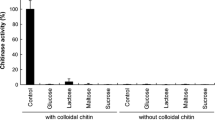
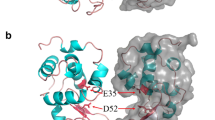
Abstract
A novel chitinase (LpChiA) was purified to homogeneity from a culture of Laceyella putida JAM FM3001. LpChiA hydrolyzed colloidal chitin optimally at a pH of 4 in an acetate buffer and temperature of 75 ºC. The enzyme was remarkably stable to incubation at 70 ºC up to 1 h at pH 5.2, and its activity half-life was 3 days. The molecular mass of the enzyme was around 38 kDa by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and around 75 kDa by gel filtration, suggesting it is a homodimer. The enzyme activity was enhanced about 60 % when pre-incubated with anionic, cationic, and nonionic surfactants. The gene for LpChiA was cloned by PCR and sequenced. The nucleotide sequence of the gene consisted of 1,683 bp encoding 560 amino acids. The N-terminal and internal amino acid sequences of the purified LpChiA from L. putida suggested that the mature enzyme was composed of 384 amino acids after cleaving its 176 N-terminal amino acids and dimerized to express its activity. The deduced amino acid sequence of the mature enzyme showed the highest similarity to chitinase of Laceyella sacchari with 79 % identity.








Similar content being viewed by others
References
Aunpad R, Panbangred W (2003) Cloning and characterization of the constitutively expressed chitinase C gene from a marine bacterium, Salinivibrio costicola strain 5SM-1. J Biosci Bioeng 96:529–536
Bassler BL, Gibbons PJ, Yu C, Roseman S (1991) Chitin utilization by marine bacteria. J Biol Chem 266:24268–24275
Bhushan B (2000) Production and characterization of a thermostable chitinase from a new alkalophilic Bacillus sp. BG-11. J Appl Microbiol 88:800–808
Christodoulou E, Duffner F, Vorgias CE (2001) Overexpression, purification, and characterization of a thermostable chitinase (Chi40) from Streptomyces thermoviolaceus OPC-520. Protein Expr Purif 23:97–105
Cohen-Kupiec R, Chet I (1998) The molecular biology of chitin digestion. Curr Opinion Biotechnol 9:270–277
Dahiya N, Tewari R, Hoondal GS (2006) Biotechnological aspects of chitinolytic enzymes: a review. Appl Microbiol Biotechnol 71:773–782
Durkin CA, Mock T, Armbrust EV (2009) Chitin in diatoms and its association with the cell wall. Eukaryot Cell 8:1038–1050
Felse PA, Panda T (2000) Production of microbial chitinases—a revisit. Bioprocess Eng 23:127–134
Fukada Y, Koide O, Miura T, Kobayashi T, Inoue A, Horikoshi K (2011) Endo-1,5-α-L-arabinase from a subseafloor Bacillus subtilis: purification, characterization, and nucleotide sequence of its gene. J Appl Glycosci 58:61–66
Gentile F, Amodeo P, Febbraio F, Picaro F, Motta A, Formisano S, Nucci R (2002) SDS-resistant active and thermostable dimmers are obtained from the dissociation of homotetrameric β-glycosidase from hyperthermophilic Sulfolobus solfataricus in SDS. J Biol Chem 277:44050–44060
Gomes RC, Semedo LT, Soares RM, Linhares LF, Ulhoa CJ, Alviano CS, Coelho RR (2001) Purification of a thermostable endochitinase from Streptomyces RC1071 isolated from a cerrado soil and its antagonism against phytopathogenic fungi. J Appl Microbiol 90:653–661
Goodsell DS, Olson AJ (2000) Structual symmetry and protein function. Annu Rev Biophys Biomol Struct 29:105–153
Hobel CFV, Hreggvidsson GÓ, Marteinsson VT, Bahrani-Mougeot F, Einarsson JM, Kristjánsson JK (2005) Cloning, expression, and characterization of a highly thermostable family 18 chitinase from Rhodothermus marinus. Extremophiles 9:53–64
Horn SJ, Sørlie M, V-Kolstad G, Norberg AL, Synstad B, Vårum KM, Eijsink VGH (2006) Comparative studies of chitinases A, B, and C from Serratia marcescens. Biocat Biotransform 24:39–53
Howard MB, Ekborg NA, Taylor LE II, Weiner RM, Hutcheson SW (2004) Chitinase B of “Microbulbifer degradans” 2–40 contains two catalytic domains with different chitinolytic activities. J Bacteriol 186:1297–1303
Hung TH, Chang YM, Sung HY, Chang CT (2002) Purification and characterization of hydrolase with chitinase and chitosanase activity from commercial stem bromelain. J Agric Food Chem 50:4666–4673
Ito S, Kobayashi T, Hatada Y, Horikoshi K (2005) Enzymes in modern detergents. Methods Biotechnol 17:151–163
Keyhani NO, Roseman S (1999) Physiological aspects of chitin catabolism in marine bacteria. Biochim Biophys Acta 1473:108–122
Kitamura E, Kamei Y (2003) Molecular cloning, sequencing and expression of the gene encoding a novel chitinase A from a marine bacterium, Pseudomonas sp. PE2, and its domain structure. Appl Microbiol Biotechnol 61:140–149
Kobayashi T, Koide O, Mori K, Shimamura S, Matsuura T, Miura T, Takaki Y, Morono Y, Nunoura T, Imachi H, Inagaki F, Takai K, Horikoshi K (2008) Phylogenetic and enzymatic diversity of deep subseafloor aerobic microorganisms in organics- and methane-rich sediments off Shimokita Peninsula. Extremophiles 12:519–527
Kobayashi T, Koide O, Deguchi S, Horikoshi K (2011) Characterization of chitosanase of a deep biosphere Bacillus strain. Biosci Biotechnol Biochem 75:669–673
Koga Y, Morikawa M, Haruki M, Nakamura H, Imanaka T, Kanaya S (1998) Thermostable glycerol kinase from a hyperthermophilic archaeon: gene cloning and characterization of the recombinant enzyme. Protein Eng 11:1219–1227
Lacey J, Cross T (1989) Genus Thermoactinomyces Tsiklinsky 1899, 501AL. In Williams VM, Sharpe E, Holt JG (eds) Bergey’s manual of systematic bacteriology vol 4 The Williams & Wilkins Co., Baltimore, pp 2574–2585
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li S, Zhao ZA, Li M, Gu ZR, Bai C, Huang WD (2002) Purification and characterization of a novel chitinase from Bacillus brevis. Acta Biochim Biophys Sin 34:690–696
Lonhienne T, Mavromatis K, Vorgias CE, Buchon L, Gerday C, Bouriotis V (2001) Cloning, sequences, and characterization of two chitinase genes from the Antarctic Arthrobacter sp. strain TAD20: isolation and partial characterization of the enzymes. J Bacteriol 183:1773–1779
Magyar C, Szilagyi A, Zivodszkyl P (1996) Relationship between thermal stability and 3-D structure in a homology model of 3-isopropylmalate dehydrogenase from Escherichia coli. Protein Eng 9:663–670
Roberts WK, Selitrennikoff CP (1988) Plant and bacterial chitinases differ in antifungal activity. J Gen Microbiol 134:169–176
Souza CP, Almeida BC, Colwell RR, Rivera ING (2011) The importance of chitin in the marine environment. Mar Biotechnol 13:823–830
Suzuki K, Taiyoji M, Sugawara N, Nikaidou N, Henrissat B, Watanabe T (1999) The third chitinase gene (chiC) of Serratia marcescens 2170 and the relationship of its product to other bacterial chitinases. Biochem J 343:587–596
Synstad B, V-Kolstad G, Cederkvist FH, Saua SF, Horn SJ, Eijsink VGH, Sørlie M (2008) Expression and characterization of endochitinase C from Serratia marcescens BJL200 and its purification by a one-step general chitinase purification method. Biosci Biotechnol Biochem 72:715–723
Takayanagi T, Ajisaka K, Takiguchi Y, Shimahara K (1991) Isolation and characterization of thermostable chitinases from Bacillus licheniformis X-7u. Biochim Biophys Acta 1078:404–410
Techkarnjanaruk S, Pongpattanakitshote S, Goodman AE (1997) Use of a promoterless lacZ gene insertion to investigate chitinase gene expression in the marine bacterium Pseudoalteromonas sp. strain S9. Appl Environ Microbiol 63:2989–2996
Tsujibo H, Yoshida Y, Miyamoto K, Imada C, Okami Y, Inamori Y (1992) Purification, properties, and partial amino acid sequence of chitinase from a marine Alteromonas sp. strain O-7. Can J Microbiol 38:891–897
van Aalten DMF, Komander D, Synstad B, Gåseidnes S, Peter MG, Eijsink VGH (2001) Structural insights into the catalytic mechanism of a family 18 exo-chitinase. Proc Natl Acad Sci 98:8979–8984
Villeret V, Clantin B, Tricot C, Legrain C, Roovers M, Stalon V, Glansdorff N, Beeumen JV (1998) The crystal structure of Pyrococcus furiosus ornithin carbamoyltransferase reveals a key role for oligomerization in enzyme stability at extremely high temperature. Proc Natl Acad Sci U S A 95:2801–2806
Watanabe T, Kobori K, Miyashita K, Fujii T, Sakai H, Uchida M, Tanaka H (1993) Identification of glutamic acid 204 and aspartic acid 200 in chitinase A1 of Bacillus circulans WL-12 as essential residues for chitinase activity. J Biol Chem 268:18567–18572
Yoon JH, Kim IG, Shint YK, Park YH (2005) Proposal of the genus Thermoactinomyces sensu stricto and three new genera, Laceyella, Thermoflavimicrobium and Seinonella, on the basis of phenotypic, phylogenetic and chemotaxonomic analyses. Int J Syst Evol Microbiol 55:395–400
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shibasaki, H., Uchimura, K., Miura, T. et al. Highly thermostable and surfactant-activated chitinase from a subseafloor bacterium, Laceyella putida . Appl Microbiol Biotechnol 98, 7845–7853 (2014). https://doi.org/10.1007/s00253-014-5692-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5692-9