Abstract
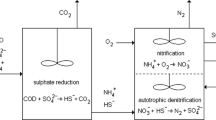
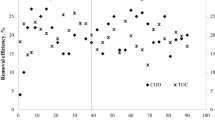
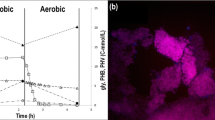
Seawater toilet flushing, seawater intrusion in the sewerage, and discharge of sulfate-rich industrial effluents elevates sulfate content in wastewater. The application of sulfate-reducing bacteria (SRB) in wastewater treatment is very beneficial; as for example, it improves the pathogen removal and reduces the volume of waste sludge, energy requirement and costs. This paper evaluates the potential to apply biological sulfate reduction using acetate and propionate to saline sewage treatment in moderate climates. Long-term biological sulfate reduction experiments at 10 and 20 °C were conducted in a sequencing batch reactor with synthetic saline domestic wastewater. Subsequently, acetate and propionate (soluble organic carbon) conversion rate were determined in both reactors, in the presence of either or both fatty acids. Both acetate and propionate consumption rates by SRB were 1.9 times lower at 10 °C than at 20 °C. At 10 °C, propionate was incompletely oxidized to acetate. At 10 °C, complete removal of soluble organic carbon requires a significantly increased hydraulic retention time as compared to 20 °C. The results of the study showed that biological sulfate reduction can be a feasible and promising process for saline wastewater treatment in moderate climate.








Similar content being viewed by others
References
Abdeen S, Di W, Hui L, Chen G-H, van Loosdrecht MCM (2010) Fecal coliform removal in a sulfate reducing autotrophic denitrification and nitrification integrated (SANI) process for saline sewage treatment. Water Sci Technol 62(11):2564–2570
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. ISBN:0-87553-223-3
Campos JL, Mosquera-Corral A, Sánchez M, Méndez R, Lema JM (2002) Nitrification in saline wastewater with high ammonia concentration in an activated sludge unit. Water Res 36(10):2555–2560
Chen G-H, Wong M-T, Okabe S, Watanabe Y (2003) Dynamic response of nitrifying activated sludge batch culture to increased chloride concentration. Water Res 37(13):3125–3135
Chen Y, Randall AA, McCue T (2004) The efficiency of enhanced biological phosphorus removal from real wastewater affected by different ratios of acetic to propionic acid. Water Res 38(1):27–36
Chen G–H, Brdjanovic D, Ekama GA, van Loosdrecht MCM (2010) Seawater as Alternative water resource. Proceedings of the 7th IWA leading edge technology conference on water and wastewater treatment, Arizona, USA, June 2–4
de Kreuk MK, Heijnen JJ, van Loosdrecht MCM (2005) Simultaneous COD, nitrogen, and phosphate removal by aerobic granular sludge. Biotechnol Bioeng 90(6):761–769
Doddema HJ, Vogels GD (1978) Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol 36(5):752–754
Harada H, Uemura S, Momonoi K (1994) Interaction between sulfate-reducing bacteria and methane-producing bacteria in UASB reactors fed with low strength wastes containing different levels of sulfate. Water Res 28(2):355–367
Henze M, Van Loosdrecht MCM, Ekama GA, Brdjanovic D (2008) Biological wastewater treatment: principles, modelling and design. IWA Publishing. ISBN: 1843391880
Kleerebezem R, Mendez R (2002) Autotrophic denitrification for combined hydrogen sulfide removal from biogas and post-denitrifcation. Water Sci Technol 45(10):349–356
Knoblauch C, Sahm K, Jorgensen BB (1999) Psychrophilic sulfate-reducing bacteria isolated from permanently cold arctic marine sediments: description of Desulfofrigus oceanense gen. nov., sp.nov., Desulfofrigus fragile sp.nov., Desulfofabe gelida gen. nog., sp. nov., Desulfotalea psychophilia gen. nov., sp. nov. and Desulfotalea arctica sp. nov. Int J Syst Bacteriol 49:1631–1643
Laanbroek HJ, Geerligs HJ, Sijtsma L, Veldkamp H (1984) Competition for sulfate and ethanol among Desulfobacter, Desulfobulbus, and Desulfovibrio species isolated from intertidal sediments. Appl Environ Microbiol 47(2):329–334
Lau GN, Sharma KR, Chen G-H, van Loosdrecht MCM (2006) Integration of sulphate reduction, autotrophic denitrification and nitrification to achieve low-cost excess sludge minimisation for Hong Kong sewage. Water Sci Technol 53(3):227–235
López-Vázquez CM, Hooijmans CM, Brdjanovic D, Gijzen HJ, van Loosdrecht MCM (2008) Factors affecting the microbial populations at full-scale enhanced biological phosphorus removal (EBPR) wastewater treatment plants in The Netherlands. Water Res 42(10–11):2349–2360
Loy A, Kusel K, Lehner A, Drake HL, Wagner M (2004) Microarray and functional gene analyses of sulfate-reducing prokaryotes in low-sulfate, acidic fens reveal cooccurrence of recognized genera and novel lineages. Appl Environ Microbiol 70(12):6998–7009
Lu H, Wu D, Tang DTW, Chen G-H, van Loosdrecht MCM, Ekama GA (2011) Pilot scale evaluation of SANI process for sludge minimization and greenhouse gas reduction in saline sewage treatment. Water Res 63(10):2149–2154
Lu H, Ekama GA, Wu D, Feng J, van Loosdrecht MCM, Chen G-H (2012) SANI® process realizes sustainable saline sewage treatment: steady state model-based evaluation of the pilot-scale trial of the process. Water Res 46(2):475–490
Mizuno O, Li YY, Noike T (1994) Effects of sulfate concentration and sludge retention time on the interaction between methane production and sulfate reduction for butyrate. Water Sci Technol 30(8):45–54
Moussa MS, Sumanasekera DU, Ibrahim SH, Lubberding HJ, Hooijmans CM, Gijzen HJ, van Loosdrecht MCM (2006) Long term effects of salt on activity, population structure and floc characteristics in enriched bacterial cultures of nitrifiers. Water Res 40(7):1377–1388
Omil F, Bakker CD, Pol LWH, Lettinga G (1997) Effect of pH and low temperature shocks on the competition between sulphate reducing bacteria and methane producing bacteria in UASB reactors. Environ Technol 18(3):255–264
Omil F, Lens P, Visser A, Hulshoff Pol LW, Lettinga G (1998) Long-term competition between sulfate reducing and methanogenic bacteria in UASB reactors treating volatile fatty acids. Biotechnol Bioeng 57(6):676–685
Oude Elferink SJWH, Visser A, Hulshoff Pol LW, Stams AJM (1994) Sulfate reduction in methanogenic bioreactors. FEMS Microbiol Rev 15(2–3):119–136
Poinapen J, Ekama G, Wentzel MC (2009) Biological sulphate reduction with primary sewage sludge in an upflow anaerobic sludge bed (UASB) reactor: Part 2. Modification of simple wet chemistry analytical procedures to achieve COD and S mass balances. Water SA 35(5):525–534
Rabus R, Brüchert V, Amann J, Könneke M (2002) Physiological response to temperature changes of the marine, sulfate-reducing bacterium Desulfobacterium autotrophicum. FEMS Microbiol Ecol 42(3):409–417
Sahinkaya E (2009) Microbial sulfate reduction at low (8°C) temperature using waste sludge as a carbon and seed source. Int Biodeterior Biodegrad 63(3):245–251
Santillano D, Boetius A, Ramette A (2010) Improved dsrA-based terminal restriction fragment length polymorphism analysis of sulfate-reducing bacteria. Appl Environ Microbiol 76(15):5308–5311
Shao M-F, Zhang T, Fang HH-P, Li X (2011) The effect of nitrate concentration on sulfide-driven autotrophic denitrification in marine sediment. Chemosphere 83(1):1–6
Tsang WL, Wang J, Lu H, Li S, Chen G-H, van Loosdrecht MCM (2009) A novel sludge minimized biological nitrogen removal process for saline sewage treatment. Water Sci Technol 59(10):1893–1899
Vaiopoulou E, Melidis P, Aivasidis A (2005) Sulfide removal in wastewater from petrochemical industries by autotrophic denitrification. Water Res 39(17):4101–4109
Vallero MVG, Sipma J, Lettinga G, Lens PNL (2004) High-rate sulfate reduction at high salinity (up to 90 mS.cm-1) in mesophilic UASB reactors. Biotechnol Bioeng 86(2):226–235
Wagner M, Roger AJ, Flax JL, Brusseau GA, Stahl DA (1998) Phylogeny of dissimilatory sulfite reductases supports an early origin of sulfate respiration. J Bacteriol 180(11):2975–2982
Wang J, Lu H, Chen G-H, Lau GN, Tsang WL, van Loosdrecht MCM (2009) A novel sulfate reduction, autotrophic denitrification, nitrification integrated (SANI) process for saline wastewater treatment. Water Res 43(9):2363–2372
Widdel, F (1988) The dissimilatory sulfate and sulfur reducing bacteria. In: Balows A, Truper HG, Dworkin M., Harder W, Schleiferk H (eds), The prokaryotes, 2nd edn. Springer; 1992. pp 583–664
Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol 129:395–400
Zhang M, Zhang T, Shao MF, Fang HHP (2009) Autotrophic denitrification in nitrate-induced marine sediment remediation and Sulfurimonas denitrificans-like bacteria. Chemosphere 76(5):677–682
Zverlov V, Klein M, Lucker S, Friedrich MW, Kellermann J, Stahl DA, Loy A, Wagner M (2005) Lateral gene transfer of dissimilatory (bi)sulfite reductase revisited. J Bacteriol 187(6):2203–2208
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 98 kb)
Rights and permissions
About this article
Cite this article
van den Brand, T.P.H., Roest, K., Brdjanovic, D. et al. Temperature effect on acetate and propionate consumption by sulfate-reducing bacteria in saline wastewater. Appl Microbiol Biotechnol 98, 4245–4255 (2014). https://doi.org/10.1007/s00253-013-5482-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5482-9