Abstract
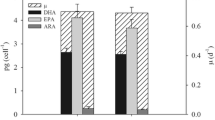
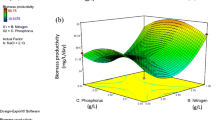
Different pilot-scale outdoor photobioreactors using medium recycling were operated in a greenhouse under different environmental conditions and the growth rates (0.1 to 0.5 day−1) obtained evaluated in order to compare them with traditional systems used in aquaculture. The annualized volumetric growth rate for Nannochloropsis gaditana was 0.26 g l−1 day−1 (peak 0.4 g l−1 day−1) at 0.4 day−1 in a 5-cm wide flat-panel bioreactor (FP-PBR). The biomass productivity achieved in this reactor was 10-fold higher than in traditional reactors, reaching values of 28 % and 45 % dry weight (d.w.) of lipids and proteins, respectively, with a 4.3 % (d.w.) content of eicosapentaenoic acid (EPA). A model for predicting EPA productivity from N. gaditana cultures that takes into account the existence of photolimitation and photoinhibition of growth under outdoor conditions is presented. The effect of temperature and average irradiance on EPA content is also studied. The maximum EPA productivity attained is 30 mg l−1 day−1.





Similar content being viewed by others
References
Acién FG, Fernández JM, Magán JJ, Molina E (2012) Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol Advances 30:1344–1353
Acién-Fernández FG, García-Camacho F, Sánchez-Pérez JA, Fernández-Sevilla JM, García-Camacho F, Molina-Grima E (2000) Modeling of eicosapentaenoic acid (EPA) production from Phaeodactylum tricornutum cultures in tubular photobioreactors. Effects of dilution rate, tube diameter, and solar irradiance. Biotechnol Bioeng 68:173–183
Aragão C, Conceição L, Dinis M, Fyhn HJ (2004) Amino acid pools of rotifers and Artemia under different conditions: nutritional implications for fish larvae. Aquaculture 234:429–445
Brown MR, Jeffrey SW, Garland CD (1989) Nutritional aspects of microalgae used in mariculture: a literature review. CSIRO Marine Reports 250
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Chini Zitelli G, Lavista F, Bastianini A, Rodolfi L, Vincenzini M, Tredici MR (1999) Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J Biotechnol 70:299–312
Del Río E, Acién FG, García-Malea MC, Rivas J, Molina-Grima E, Guerrero MG (2005) Efficient one-step production of astaxanthin by the microalga Haematococcus pluvialis in continuous culture. Biotechnol Bioeng 91:808–815
Fábregas J, Otero A, Morales ED, Arredondo-Vega BO, Patiño M (1998) Modification of the nutritive value of Phaeodactylum tricornutum for Artemia sp. in semicontinuous cultures. Aquaculture 169:167–176
Figueroa FL, Jiménez C, Lubián LM, Montero O, Lebert M, Häder DP (1997) Effects of high irradiance and temperature on photosynthesis and photoinhibition in Nannochloropsis gaditana Lubián (Eustigmatophyceae). J Plant Physiol 151:6–15
González-López CV, Cerón-García MC, Fernández-Sevilla JM, González-Céspedes AM, Camacho-Rodríguez J, Molina-Grima E (2013) Medium recycling for Nannochloropsis gaditana cultures for aquaculture. Bioresour Technol 129:430–438
Hu C, Li M, Li J, Zhu Q, Liu Z (2007) Variation of lipid and fatty acid compositions of the marine microalga Pavlova viridis (Prymnesiophyceae) under laboratory and outdoor culture conditions. W J Microbiol Biotechnol 24:1209–1214
Kochert G (1978) Quantitation of the macromolecular components of microalgae. In: Hellebust JA, Craigie JS (eds) Handbook of phycological methods, physiological and biochemical methods. Cambridge University Press, Cambridge, pp 189–195
López CV, Cerón MC, Acién FG, Segovia C, Chisti Y, Fernández JM (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101:7587–7591
Lubián LM, Montero O, Moreno-Garrido I, Huertas IE, Sobrino C, González-Del Valle M, Parés G (2000) Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J Appl Phycol 12(3–5):249–255
Lynch DV, Thompson GA (1982) Low temperature-induced alterations in the chloroplast and microsomal-membranes of Dunaliella salina. Plant Physiol 69:1369–1375
Molina Grima E, García Camacho F, Sánchez Pérez JA, Fernández Sevilla JM, Acién Fernández FG, Contreras Gómez A (1994) A mathematical model of microalgal growth in light-limited chemostat culture. J Chem Technol Biotechnol 61:167–173
Molina Grima E, García Camacho F, Sánchez Pérez JA, Acién Fernández FG, Fernández Sevilla JM (1997) Evaluation of photosynthetic efficiency in microalgal cultures using averaged irradiance. Enzyme Microb Tech 21(5):375–381
Muller-Feuga A (2000) The role of microalgae in aquaculture: situation and trends. J Appl Ichthyol 12:527–534
Otero A, Patiño M, Domínguez A, Fábregas J (2002) Tailoring the nutritional composition of microalgae for aquaculture purposes — the use of semicontinuos culture techniques. Aquac Eur 33:13–16
Piña P, Voltolina D, Nieves M, Robles M (2006) Survival, development and growth of the Pacific white shrimp Litopenaeus vannamei protozoea larvae, fed with monoalgal and mixed diets. Aquaculture 253:523–530
Quinn JC, Yates T, Douglas N, Weyer K, Butler J, Bradley TH, Lammers PJ (2012) Nannochloropsis production metrics in a scalable outdoor photobioreactor for commercial applications. Bioresour Technol 117:164–171
Renaud SM, Parry DL, Luong-Van T, Kuo C, Padovan A, Sammy N (1991) Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochlropsis oculata for use in tropical aquaculture. J Appl Phycol 3:43–53
Renaud SM, Thinh LV, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211:195–214
Rocha JMS, García JEC, Henriques MHF (2003) Growth aspects of the marine microalga Nannochloropsis gaditana. Biomol Engin 20:237–242
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Rodríguez-Ruiz J, Belarbi E, Sánchez JLG, Alonso DL (1998) Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnol Techniques 12:689–691
San Pedro A, González-López CV, Acién FG, Molina-Grima E (2013) Marine microalgae selection and culture conditions optimization for biodiesel production. Bioresource Technol 134:353–361
Sánchez JF, Fernández-Sevilla JM, Acién FG, Cerón MC, Pérez-Parra J, Molina-Grima E (2008) Biomass and lutein productivity of Scenedesmus almeriensis: influence of irradiance, dilution rate and temperature. Appl Microbiol Biotechnol 79:719–729
Seto A, Wang HL, Hesseltine CW (1984) Culture conditions affect eicosapentaenoic acid content of Chlorella minutissima. J Am Oil Chem Soc 61:892–894
Seto A, Kumasaka K, Hosaka M, Kojima E, Kashiwakura M, Kato T (1992) Production of eicosapentaenoic acid by marine microalgae and its commercial utilization for aquaculture. In: Kyle DJ, Ratledge C (eds) Industrial application of single cell oils. American Oil Chemists’ Society, Champaign, IL, pp 219–234
Sierra E, Acién FG, Fernández JM, García JL, González C, Molina E (2008) Characterization of a flat plate photobioreactor for the production of microalgae. Chem Eng J 138:136–147
Simionato D, Sforza E, Corteggiani Carpinelli E, Bertucco A, Giacometti GM, Morosinotto T (2011) Acclimatation of Nannochloropsis gaditana to different illumination regimes: Effects on lipids accumulation. Bioresource Technol 102:6026–6032
Sukenik A (1991) Ecophysiological considerations in the optimization of eicosapentaenoic acid production by Nannochloropsis sp. (Eustigmatophyceae). Bioresour Technol 35:263–269
Sukenik A, Zmora O, Carmeli Y (1993a) Biochemical quality of marine unicellular algae with special emphasis on lipid composition. Aquaculture 117:313–326
Sukenik A, Yamaguchi Y, Livne A (1993b) Alterations in lipid molecular species of the marine eustigmatophyte Nannochloropsis sp. J Phycol 29:620–626
Teshima S, Yamasaki S, Kanazawa A, Hirata H (1983) Effects of water temperature and salinity on eicosapentaenoic acid level of marine Chlorella. Bull Jpn Soc Sci Fisheries 49:805
Thompson PA, Guo MX, Harrison PJ, Whyte JNC (1992) Effects of variation in temperature. On the fatty-acid composition of 8 species of marine-phytoplankton. J Phycol 28:488–497
Torzillo G, Pushparaj B, Bocci F, Balloni W, Materassi R, Florenzano G (1986) Production of Spirulina biomass in closed photobioreactors. Biomass 11:61–74
Volkman JK, Malcom RB, Dunstan GA, Jeffrey SW (1993) The biochemical composition of marine microalgae from the class Eustigmatophiceae. J Phycol 29:69–78
Wahidin S, Idris A, Muhamad Shaleh SR (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresource Technol 129:7–11
Zou N, Richmond A (1999) Effect of light-path length in outdoor flat plate reactors on output rate of cell mass and of EPA in Nannochloropsis sp. J Biotechnol 70:351–356
Acknowledgements
This research was supported by the General Secretariat of Universities, Research and Technology of the Andalusian Government (AGR-5334) and was co-financed by the European Regional Development Fund (ERDF). We would also like to thank CAJAMAR Foundation for its support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camacho-Rodríguez, J., González-Céspedes, A.M., Cerón-García, M.C. et al. A quantitative study of eicosapentaenoic acid (EPA) production by Nannochloropsis gaditana for aquaculture as a function of dilution rate, temperature and average irradiance. Appl Microbiol Biotechnol 98, 2429–2440 (2014). https://doi.org/10.1007/s00253-013-5413-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5413-9