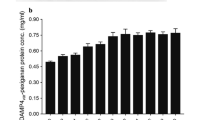
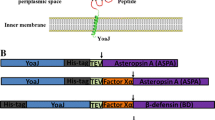
Abstract
Human α-defensin 6 (HD6), a small cysteine-rich cationic peptide specially expressed in epithelial cells of digestive tract, may play a crucial role in mucosal immunity. This is the first report on efficient production of bioactive HD6 through a gene-engineering approach in Escherichia coli. The recombinant plasmid pET32a-omHD6 was primarily constructed by inserting a PCR fragment encoding mature HD6 peptide (mHD6) preceded by an enterokinase recognition sequence into the expression vector pET32a(+), in frame with the upstream thioredoxin (TrxA) gene. Under optimized expression conditions, a high percentage (>60%) of soluble TrxA-omHD6 fusion protein was obtained with a yield of about 1.69 g/l, and the theoretical productivity of recombinant mHD6 (rmHD6) reached 0.38 g/l. A feasible three-step purification strategy involving nickel-sepharose chromatography, enterokinase-cleavage and cation exchange chromatography was developed to purify rmHD6, followed by characteristic identifications by Western blot, mass spectrometry and sequencing. About 102 mg/l of rmHD6 with its intact N-terminal amino acid sequence was finally achieved. The in vitro experiments showed that rmHD6 possesses high potency to inhibit herpes simplex virus-2 infection. This work settles substantial foundation for further functional study of HD6.






Similar content being viewed by others
References
Ario de Marco (2009) Strategies for successful recombinant expression of disulfide bond-dependent proteins in Escherichia coli. Microb Cell Factories 26:1–18
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT (2006) Human α-defensins block papillomavirus infection. Proc Natl Acad Sci USA 103:1516–1521
Chen H, Xu Z, Peng L, Fang X, Yin X, Xu N, Cen P (2006) Recent advances in the research and development of human defensins. Peptides 27:931–940
Cipakova I, Gasperik J, Hostinova E (2006) Expression and purification of human antimicrobial peptide, dermcidin, in Escherichia coli. Protein Expr Purif 45:269–274
Ericksen B, Wu Z, Lu W, Lehrer RI (2005) Antibacterial activity and specificity of the six human α-defensins. Antimicrob Agents Chemother 49:269–275
Frye M, Bargon J, Dauletbaev N, Weber A, Wagner TO, Gropp R (2000) Expression of human α-defensin (HD5) mRNA in nasal and bronchial epithelial cells. J Clin Pathol 53:770–773
Hazrati E, Galen B, Lu W, Wang W, Ouyang Y, Keller MJ, Lehrer RI, Herold BC (2006) Human α- and β-defensins block multiple steps in herpes simplex virus infection. J Immunol 177:8658–8666
Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR (1989) Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68
Jones DE, Bevins CL (1992) Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem 267:23216–23225
Jones DE, Bevins CL (1993) Defensin 6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett 315:187–192
Lee JH, Minn I, Park CB, Kim SC (1998) Acidic peptide-mediated expression of the antimicrobial peptide buforin II as tandem repeats in Escherichia coli. Protein Expr Purif 12:53–60
Leeuw E, Burks SR, Li X, Kao JP, Lu W (2007) Structure-dependent functional properties of human defensin 5. FEBS Lett 581:515–520
Nam MJ, Kee MK, Kuick R, Hanash SM (2005) Identification of α-defensin 6 as a potential biomarker in colon adenocarcinoma. J Biol Chem 280:8260–8265
Ouellette AJ (2006) Paneth cell α-defensin synthesis and function. Curr Top Microbiol Immunol 306:1–25
Peng L, Xu Z, Fang X, Wang F, Yang S, Cen P (2004) Preferential codons enhancing the expression level of human β-defensin-2 in recombinant Escherichia coli. Protein Pept Lett 11:339–344
Porter EM, van Dam E, Valore EV, Ganz T (1997) Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun 65:2396–2401
Porter EM, Poles MA, Lee JS, Naitoh J, Bevins CL, Ganz T (1998) Isolation of human intestinal defensins from ileal neobladder urine. FEBS Lett 434:272–276
Prinz WA, Aslund F, Holmgren A, Beckwith J (1997) The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J Biol Chem 272:15661–15667
Rao XC, Li S, Hu JC, Jin XL, Hu XM, Huang JJ, Chen ZJ, Zhu JM, Hu FQ (2004) A novel carrier molecule for high-level expression of peptide antibiotics in Escherichia coli. Protein Expr Purif 36:11–18
Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL (2003) Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522–526
Tian ZG, Dong TT, Yang YL, Teng D, Wang JH (2009) Expression of antimicrobial peptide LH multimers in Escherichia coli C43(DE3). Appl Microbiol Biotechnol 83:143–149
Wang A, Wang S, Shen M, Chen F, Zou Z, Ran X, Cheng T, Su Y, Wang J (2009) High level expression and purification of bioactive human α-defensin 5 mature peptide in Pichia pastoris. Appl Microbiol Biotechnol 84:877–884
Wehkamp J, Chu H, Shen B, Feathers RW, Kays RJ, Lee SK, Bevins CL (2006) Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett 580:5344–5350
Wei Q, Kim YS, Seo JH, Jang WS, Lee IH, Cha HJ (2005) Facilitation of expression and purification of an antimicrobial peptide by fusion with baculoviral polyhedrin in Escherichia coli. Appl Environ Microbiol 71:5038–5043
Wu Z, Ericksen B, Tucker K, Lubkowski J, Lu W (2004) Synthesis and characterization of human α-defensins 4-6. J Pept Res 64:118–125
Xu X, Jin F, Yu X, Ji S, Wang J, Cheng H, Wang C, Zhang W (2007) Expression and purification of a recombinant antibacterial peptide, cecropin, from Escherichia coli. Protein Expr Purif 53:293–301
Zhong Z, Xu Z, Peng L, Huang L, Fang X, Cen P (2006) Tandem repeat mhBD2 gene enhance the soluble fusion expression of hBD2 in Escherichia coli. Appl Microbiol Biotechnol 71:661–667
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 30771892), Academician Fund of Chongqing City (No. CSTC, 2007AB5022), Special Fund of National Key Laboratory of Trauma, Burn and Combined Injury (No. SKLZZ200821), and National “863” High-tech Development Plan of China (2007AA02Z152).
Author information
Authors and Affiliations
Corresponding author
Elecronic Supplementary Materials
Below is the link to the electronic supplementary material.
Fig. S1
Purified rmHD6 analyzed by reversed phase high performance liquid chromatography. The purity of rmHD6 is 74.7% (GIF 13 kb)
Fig. S2
Purified rmHD6 and Dithiothreitol-reduced rmHD6 analyzed by MALDI-TOF mass spectrometry. Molecular mass of rmHD6 is 3,707.6 Da (a), and reduced rmHD6 is 3713.4 Da (b) (GIF 34 kb)
Rights and permissions
About this article
Cite this article
Wang, A., Su, Y., Wang, S. et al. High efficiency preparation of bioactive human α-defensin 6 in Escherichia coli Origami(DE3)pLysS by soluble fusion expression. Appl Microbiol Biotechnol 87, 1935–1942 (2010). https://doi.org/10.1007/s00253-010-2688-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2688-y