Abstract
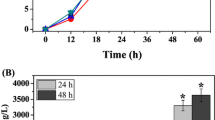
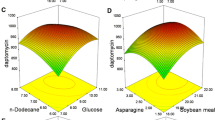
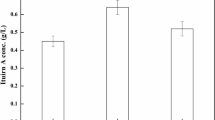
Phenazine-1-carboxylic acid (PCA) production was enhanced in Pseudomonas sp. M18 wild strain and its mutants carrying recombinant pME6032Phz for phz gene cluster overexpression, among which Pseudomonas sp. strain M18GQ/pME6032Phz, a gacA and qscR double gene chromosomally inactivated mutant harboring pME6032Phz, showed the highest PCA yield. The conditions for fermentation and isopropyl-β-d-1-thiogalactopyranoside (IPTG) induction were optimized for strain M18GQ/pME6032Phz in shake flask experiments. A one-factor-at-a-time approach, followed by a fractional factorial design identified soybean meal, corn steep liquor, and ethanol as statistically significant factors. Optimal concentrations and mutual interactions of the factors were then determined by the method of steepest ascent and by response surface methodology based on the center composite design. The predicted PCA production was 6,335.2 mg/l after 60 h fermentation in the optimal medium of 65.02 g soybean meal, 15.36 g corn steep liquor, 12 g glucose, 21.70 ml ethanol, and 1 g MgSO4 per liter in the flask fermentations, with induction of 1.0 mmol/l IPTG 24 h after inoculation. In an experimental validation under these conditions, the maximum PCA production was 6,365.0 mg/l. This represents a ∼60% increase over production by strain M18GQ in optimal conditions. The negative effect of plasmid pME6032 on the expression of chromosomally located phz gene cluster was found in Pseudomonas sp. M18GQ, and the possible reason was discussed in the text.




Similar content being viewed by others
References
Butler MJ, Takano E, Bruheim P, Jovetic S, Marinelli F, Bibb MJ (2003) Deletion of scbA enhances antibiotic production in Streptomyces lividans. Appl Microbiol Biotechnol 61:512–516
Daly R, Hearn MT (2005) Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J Mol Recognit 18:119–138
Deepak V, Kalishwaralal K, Ramkumarpandian S, Babu SV, Senthilkumar SR, Sangiliyandi G (2008) Optimization of media composition for Nattokinase production by Bacillus subtilis using response surface methodology. Bioresour Technol 99:8170–8174
Donovan RS, Robinson CW, Glick BR (2000) Optimizing the expression of a monoclonal antibody fragment under the transcriptional control of the Escherichia coli lac promoter. Can J Microbiol 46:532–541
Ge Y, Huang X, Wang S, Zhang X, Xu Y (2004) Phenazine-1-carboxylic acid is negatively regulated and pyoluteorin positively regulated by gacA in Pseudomonas sp. M18. FEMS Microbiol Lett 237:41–47
Gibson J, Sood A, Hogan DA (2009) Pseudomonas aeruginosa-Candida albicans interactions: localization and fungal toxicity of a phenazine derivative. Appl Environ Microbiol 75:504–513
Graupner S, Wackernagel W (2000) A broad-host-range expression vector series including a Ptac test plasmid and its application in the expression of the dod gene of Serratia marcescens (coding for ribulose-5-phosphate 3-epimerase) in Pseudomonas stutzeri. Biomol Eng 17:11–16
Heeb S, Itoh Y, Nishijyo T, Schnider U, Keel C, Wade J, Walsh U, O’Gara F, Haas D (2000) Small, stable shuttle vectors based on the minimal pVS1 replicon for use in Gram-negative, plant-associated bacteria. Mol Plant Microb Interact 13:232–237
Hu HB, Xu YQ, Chen F, Zhang XH, Hur BK (2005) Isolation and characterization of a new fluorescent Pseudomonas strain that produces both phenazine-1-carboxylic acid and pyoluteorin. J Microbiol Biotechnol 15:86–90
Huang J, Xu Y, Zhang H, Li Y, Huang X, Ren B, Zhang X (2009) Temperature-dependent expression of phzM with its regulatory genes lasI and ptsP in rhizosphere isolated Pseudomonas sp. Strain M18. Appl Environ Microbiol 75:6568–6580
Jiang K, Pi Y, Hou R, Zeng H, Huang Z, Zhang Z, Sun X, Tang K (2009) Molecular cloning and expression profiling of the first specific jasmonate biosynthetic pathway gene allene oxide synthase from Lonicera japonica. Mol Biol Rep 36:487–493
Kennedy MM, Krouse D (1999) Strategies for improving fermentation medium performance: a review. J Ind Microbiol Biotech 23:456–475
Khuri AI, Cornell JA (1987) Response surfaces: design and analysis. Marcel Decker Inc., New York
Kumar RS, Ayyadurai N, Pandiaraja P, Reddy AV, Venkateswarlu Y, Prakash O, Sakthivel N (2005) Characterization of antifungal metabolite produced by a new strain Pseudomonas aeruginosa PUPa3 that exhibits broad-spectrum antifungal activity and biofertilizing traits. J Appl Microbiol 98:145–154
Li Y, Jiang H, Du X, Huang X, Xu Y (2010) Enhancement of phenazine-1-carboxylic acid production using batch and fed-batch culture of gacA inactivated Pseudomonas sp. M18G. Bioresour Technol (in press)
Li Y, Jiang H, Xu Y, Zhang X (2008) Optimization of nutrient components for enhanced phenazine-1-carboxylic acid production by gacA-inactivated Pseudomonas sp. M18G using response surface method. Appl Microbiol Biotechnol 77:1207–1217
Li Y, Lu J (2005) Characterization of the enzymatic degradation of arabinoxylans in grist containing wheat malt using response surface methodology. J Am Soc Brew Chem 63:171–176
Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS (2001) Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol 21:6454–6465
Mazzola M, Cook RJ, Thomashow LS, Weller DM, Pierson LS 3rd (1992) Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol 58:2616–2624
Mundra P, Desai K, Lele SS (2007) Application of response surface methodology to cell immobilization for the production of palatinose. Bioresour Technol 98:2892–2896
Muralidhar RV, Chirumamilla RR, Marchant R, Nigam P (2001) A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem Eng J 9:17–23
Raaijmakers JM, Weller DM, Thomashow LS (1997) Frequency of antibiotic-producing Pseudomonas spp. in natural environments. Appl Environ Microbiol 63:881–887
Reynard LN, Cocquet J, Burgoyne PS (2009) The multi-copy mouse gene Sycp3-like Y-linked (Sly) encodes an abundant spermatid protein that interacts with a histone acetyltransferase and an acrosomal protein. Biol Reprod 81:250–257
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sayyad SA, Panda BP, Javed S, Ali M (2007) Optimization of nutrient parameters for lovastatin production by Monascus purpureus MTCC 369 under submerged fermentation using response surface methodology. Appl Microbiol Biotechnol 73:1054–1058
Shtark O, Shaposhnikov AI, Kravchenko LV (2003) The production of antifungal metabolites by Pseudomonas chlororaphis grown on different nutrient sources. Mikrobiologiia 72:645–650
Slininger PJ, Jackson MA (1992) Nutritional factors regulating growth and accumulation of phenazine-1-carboxylic acid by Pseudomonas fluorescens 2–79. Appl Microbiol Biotechnol 37:388–392
Stock R, Grant RJ, Klopfenstein TJ (1995) Average composition of feeds used in Nebraska. Institute of Agricultural Resources, University of Nebraska Lincoln, Lincoln
Su J, Zhou Q, Zhang H, Li Y, Huang X, Xu Y (2009) Medium optimization for phenazine-1-carboxylic acid production by a chromosomally inactivated gacA and qscR double mutant Pseudomonas sp. M18GQ using response surface methodology. Bioresour Technol (in press)
Tang XJ, He GQ, Chen QH, Zhang XY, Ali MA (2004) Medium optimization for the production of thermal stable beta-glucanase by Bacillus subtilis ZJF-1A5 using response surface methodology. Bioresour Technol 93:175–181
Thomashow LS, Weller DM (1988) Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol 170:3499–3508
Thomashow LS, Weller DM, Bonsall RF, Pierson LS (1990) Production of the antibiotic phenazine-1-carboxylic acid by fluorescent Pseudomonas species in the rhizosphere of wheat. Appl Environ Microbiol 56:908–912
Wang W, Wen X (2009) Expression of lignin peroxidase H2 from Phanerochaete chrysosporium by multi-copy recombinant Pichia strain. J Environ Sci (China) 21:218–222
Wang Y, Huang X, Hu H, Zhang X, Xu Y (2008) QscR acts as an intermediate in gacA-dependent regulation of PCA biosynthesis in Pseudomonas sp. M-18. Curr Microbiol 56:339–345
Wlad H, Ballagi A, Bouakaz L, Gu Z, Janson JC (2001) Rapid two-step purification of a recombinant mouse Fab fragment expressed in Escherichia coli. Protein Expr Purif 22:325–329
Xiao ZJ, Liu PH, Qin JY, Xu P (2007) Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl Microbiol Biotechnol 74:61–68
Xiong ZQ, Tu XR, Tu GQ (2008) Optimization of medium composition for actinomycin X2 production by Streptomyces spp JAU4234 using response surface methodology. J Ind Microbiol Biotechnol 35:729–734
Yuan LL, Li YQ, Wang Y, Zhang XH, Xu YQ (2008) Optimization of critical medium components using response surface methodology for phenazine-1-carboxylic acid production by Pseudomonas sp. M-18Q. J Biosci Bioeng 105:232–237
Acknowledgments
This work was supported in part by grants from the Major State Basic Research Development Program of China (973 Program; no. 2009CB118906), the National High Technology Research and Development Program of China (863 Program; nos. 2006AA10A209 and 2006AA02Z228), and the Shanghai Science and Technology Program (no. 08391911900).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Q., Su, J., Jiang, H. et al. Optimization of phenazine-1-carboxylic acid production by a gacA/qscR-inactivated Pseudomonas sp. M18GQ harboring pME6032Phz using response surface methodology. Appl Microbiol Biotechnol 86, 1761–1773 (2010). https://doi.org/10.1007/s00253-010-2464-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2464-z