Abstract
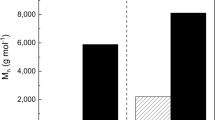
Linear copolymeric polythioesters [PTE; poly(α,ω-alkanedioic acid-co-α,ω-alkanedithiols)] were formed in good yield (∼69%) by thioesterification of 1,12-dodecanedioic acid with 1,6-hexanedithiol and 1,8-octanedithiol, respectively, catalyzed by immobilized lipase from Rhizomucor miehei (Lipozyme RM IM) in vacuo without a solvent. Similarly, transthioesterification (thiolysis) of diethyl 1,12-dodecanedioate with 1,6-hexanedithiol led to the formation of ∼66% PTE. Poly (1,12-dodecanedioic acid-co-1,6-hexanedithiol) and poly (1,12-dodecanedioic acid-co-1,8-octanedithiol) were extracted from the reaction mixture using methyl-t-butylether, precipitated at −20°C and the precipitates extracted with boiling i-hexane to yield two fractions of PTE. The i-hexane-insoluble fraction of poly (1,12-dodecanedioic acid-co-1,6-hexanedithiol) shows an average molecular mass (Mw) of 1,212 Da, corresponding to a molecular weight range of up to 13,200 Da and a degree of polymerization of up to 38 monomer units. The i-hexane-insoluble fraction of poly (1,12-dodecanedioic acid-co-1,8-octanedithiol) shows a Mw of 2,360 Da, corresponding to a molecular weight range of up to 19,500 Da and a maximum degree of polymerization of up to 52 monomer units. The low-molecular weight (<800 Da) reaction products of thioesterification of 1,12-dodecanedioic acid with 1,6-hexanedithiol, elucidated by gas chromatography–mass spectroscopy, show the following intermediates: (1) 9,20-dioxo-1,8-dithiacycloeicosane; (2) 17,28-dioxo-1,8,9,16-tetrathiacyclooctacosane; (3) 1,12-dodecanedioic acid methyl(O)ester 6′-S-mercaptohexyl thio(S)ester; and (4) oligomeric linear thioester, formed by thioesterification of two molecules of 1,12-dodecanedioic acid with one molecule of 1,6-hexanedithiol.



Similar content being viewed by others
References
Bührer HG, Elias H-G (1970) Polythiolester. II. Polythiolactid. Makromol Chem 140:41–54
Caussette M, Marty A, Combes D (1997) Enzymatic synthesis of thioesters in non-conventional solvents. J Chem Technol Biotechnol 68:257–262
Cavaille-Lefèbvre D, Combes D (1997) Lipase synthesis of short-chain flavour thioesters in solvent-free medium. Biocatal Biotransform 15:265–279
Elbanna K, Lütke-Eversloh T, van Trappen S, Mergaert J, Swings J, Steinbüchel A (2003) Schlegelella thermodepolymerans gen. nov., sp. nov., a novel thermophilic bacterium that degrades poly (3-hydroxybutyrate-co-3-mercaptopropionate). Int J Syst Evol Microbiol 53:1165–1168
Elbanna K, Lütke-Eversloh T, Jendrossek D, Luftmann H, Steinbüchel A (2004) Studies on the biodegradability of polythioester copolymers and homopolymers by polyhydroxyalkanoate (PHA)-degrading bacteria and PHA depolymerases. Arch Microbiol 182:212–225
Elias H-G, Bührer HG (1970) Polythiolester. I. Polythioglykolid. Makromol Chem 140:21–39
Guo ZW, Sih CJ (1988) Enzymic synthesis of macrocyclic lactones. J Am Chem Soc 110:1999–2001
Iwata S, Toshima K, Matsumura S (2003) Enzyme-catalyzed preparation of aliphatic polyesters containing thioester linkages. Macromol Rapid Commun 24:467–471
Kricheldorf HR (1973) Synthese und Polymerisation von 2,4-Dioxo-1,3-dithian. Makromol Chem 173:81–89
Kricheldorf HR, Bösinger K (1973) Über die Polymerisation von 2,5-Dioxo-1,3-oxathiolan und 2,4-Dioxo-1,3-dithiolan. Makromol Chem 173:67–80
Kricheldorf HR, Probst N, Schwarz G, Schulz G, Krüger R-P (2000) New polymer syntheses. 107. Aliphatic poly(thio ester)s by ring-opening polycondensation of 2-stanna-1,3-dithiacycloalkanes. J Polym Sci A Polym Chem 38:3656–3664
Lin KF (1996) Paints, varnishes, and related products. In: Hui YH (ed) Bailey's Industrial Oil and Fat Products, vol 5. Wiley-Interscience, New York, pp 227–274
Lütke-Eversloh T, Bergander K, Luftmann H, Steinbüchel A (2001) Identification of a new class of biopolymer: bacterial synthesis of a sulfur-containing polymer with thioester linkages. Microbiology 147:11–19
Lütke-Eversloh T, Fischer A, Remminghorst U, Kawada J, Marchessault RH, Bögershausen A, Kalwei M, Eckert H, Reichelt R, Liu S-J, Steinbüchel A (2002) Biosynthesis of novel thermoplastic polythioesters by engineered Escherichia coli. Nat Mater 1:236–240
Marvel CS, Kotch A (1951) Polythiolesters. J Am Chem Soc 73:1100–1102
Sanda F, Jirakanjana D, Hitomi M, Endo T (2000) Cationic ring-opening polymerization of ɛ-thionocaprolactone: selective formation of polythioester. J Polym Sci A Polym Chem 38:4057–4061
Schöberl A (1960) Über Polyglykolide. Makromol Chem 37:64–70
Spiteller G (1966) Massenspektrometrische Strukturanalyse organischer Verbindungen. Verlag Chemie, Weinheim, p 140
Steinbüchel A (2003) Production of rubber-like polymers by microorganisms. Curr Opin Microbiol 6:261–270
Uhrich KE (2003) Antibiotic polymers. World Patent WO 03/066053 A1 14 August 2003
Weber N, Klein E, Vosmann K, Mukherjee KD (1998) Preparation of long-chain acyl thioesters-thio wax esters-by the use of lipases. Biotechnol Lett 20:687–691
Weber N, Klein E, Mukherjee KD (1999) Long-chain acyl thioesters by solvent-free thioesterification and transthioesterification catalyzed by microbial lipases. Appl Microbiol Biotechnol 51:401–404
Weber N, Klein E, Vosmann K, Mukherjee KD (2000) Antioxidants eliminate stereomutation and thioether formation during lipase-catalyzed thioesterification and transthioesterification for the preparation of uniform cis- and trans-unsaturated thioesters. Chem Phys Lipids 105:215–223
Weber N, Klein E, Vosmann K, Mukherjee KD (2004) Mono-thioesters and di-thioesters by lipase-catalyzed reactions of α,ω-alkanedithiols with palmitic acid or its methyl ester. Appl Microbiol Biotechnol 64:800–805
Zaks A, Klibanov AM (1985) Enzyme-catalysed processes in organic solvents. Proc Natl Acad Sci U S A 82:3192–3196
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weber, N., Bergander, K., Fehling, E. et al. Copolymeric polythioesters by lipase-catalyzed thioesterification and transthioesterification of α,ω-alkanedithiols. Appl Microbiol Biotechnol 70, 290–297 (2006). https://doi.org/10.1007/s00253-005-027-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-027-5