Abstract
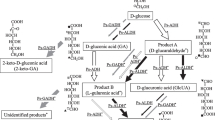
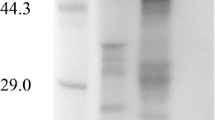
A soluble glucoside 3-dehydrogenase (G3DH) from Stenotrophomonas maltrophilia CCTCC M 204024, recently isolated from wheat soil in our laboratory, was purified to 37.4-fold with a yield of 24.7% and was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a molecular mass of 66 kDa. 2,6-Dichlorophenolindophenol (DCPIP) and ferricyanide were able to act as artificial electron acceptors for the enzyme. The optimal pH of G3DH was in the range of 6.0–7.0 in the presence of DCPIP. The enzyme was stable in the pH range of 4.4–10.6 and was sensitive to heat. G3DH exhibited extremely broad substrate specificity by converting many sugars to their corresponding 3-ketoglucosides. They produced a characteristic spectrum by alkaline treatment with a peak at 340 nm. The apparent K m values for validoxylamine A and d-glucose were 8.3 and 1.1 mM, respectively. Cu2+, Ag2+, and Hg2Cl2 inhibited the activity of G3DH.






Similar content being viewed by others
References
Appleby CA, Morton RK (1959) Lactic dehydrogenase and cytochrome b2 from yeast, purification and crystallization. J Biochem 71:492
Armstrong JM (1964) The molar extinction coefficient of 2,6-dichlorophenol indophenol. Biochim Biophys Acta 86:194–197
Asano N, Takeuchi M, Ninomiya K, Kameda Y, Matsui K (1984) Microbial degradation of validamycin A by Flavobacterium saccharophilum: enzyme cleavage of C–N linkage in validoxylamine A. J Antibiot 37(8):859–867
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Fukui S, Hayano K (1969) Micro methods for determination of 3-ketosucrose and 3-ketoglucose. Agric Biol Chem 33:1013–1017
Hamafuji T, Tsugawa W, Sode K (2002) Clinical application of the serum 1,5-anhydroglucitol assay method using glucose 3-dehydrogenase. J Clin Lab Anal 16(6):299–303
Hayano K, Fukui S (1967) Purification and properties of 3-ketosucrose-forming enzyme from the cells of Agrobacterium tumefaciens. J Biol Chem 242:3665–3672
Kojima K, Tsugawa W, Hamahuji T, Watazu Y, Sode K (1999) Effect of growth substrates on production of new soluble glucose 3-dehydrogenase in Halomonas (Deleya) sp. α-15. Appl Biochem Biotechnol 77–79:827–834
Kameda Y, Asano N, Teranishi M, Matsui K (1980) New cyclitols, degradation of validamycin A by Flavobacterium saccharophilum. J Antibiot 33:1573–1574
Kurowski WM, Fensom AH, Pirt SJ (1975) Factors influencing the formation and stability of D-glucoside 3-dehydrogenase activity in cultures of Agrobacterium tumefaciens. J Gen Microbiol 90(2):191–202
Laemmli UK (1970) Cleavage of structural proteins during the assembly of bacteriphoge T4. Nature 227:680–685
Maeda A, Adachi S, Matsuno R (2001) Improvement of selectivity in 3-ketocellobiose production from cellobiose by Agrobacterium tumefaciens. J Biol Chem 8:217–221
Morrison SC, Wood DA, Wood PM (1999) Characterization of a glucose 3-dehydrogenase from the cultivated mushroom (Agaricus bisporus). Appl Microbiol Biotechnol 51(1):58–64
Pietsch M, Walter M, Buchholz K (1994) Regioselective synthesis of new sucrose derivatives via 3-keto sucrose. Carbohydr Res 254:183
Sode K, Akaike E, Sugiura H, Tsugawa W (2001) Enzymatic synthesis of a novel trehalose derivative, 3,3′-diketoneotrehalose, and its potential application as the trehalase enzyme inhibitor. FEBS Lett 489:42–45
Takeuchi M, Ninomiya K, Kawabata K, Asano N, Kameda Y, Matsui K (1986) Purification and properties of glucoside 3-dehydrogenase from Flavobacterium saccharophilum. J Biochem 100(4):1049–1055
Takeuchi M, Asano N, Kameda Y, Matsui K (1988a) Physiological role of glucoside 3-dehydrogenase and cytochrome c551 in the sugar oxidizing system of Flavobacterium saccharophilum. J Biochem 103(6):938–943
Takeuchi M, Asano N, Kameda Y, Matsui K (1988b) Purification and properties of soluble D-glucoside 3-dehydrogenase from Flavobacterium saccharophilum. Agric Biol Chem 52(8):1905–1912
Tsugawa W, Horiuchi S, Tanaka M, Wake H, Sode K (1996) Purification of a marine bacterial glucose dehydrogenase from Cytophaga marinoflava and its application for measurement of 1,5-anhydro-D-glucitol. Appl Biochem Biotechnol 56:301–310
Zheng YG, Jin LQ, Shen YC (2004) Resin-catalyzed degradation of validamycin A for production of validoxylamine A. Catal Commun 5:519–525
Zheng YG, Shentu XP, Shen YC (2005a) Inhibition of porcine small intestinal sucrase by valienamine. J Enzyme Inhib Med Chem 20:49–53
Zheng, YG, Zhang XF, Shen YC (2005b) Microbial transformation of validamycin A to valienamine by immobilized cells. Biocatal Biotransform 23:71–77
Acknowledgements
This work was supported by the National Fund of the Major Basic Research Development Program 973 of China (2003CB716005), the National Natural Science Foundation of China (20176055), and the Natural Science Foundation of Zhejiang Province (ZB0106).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, JF., Zheng, YG., Xue, YP. et al. Purification and characterization of the glucoside 3-dehydrogenase produced by a newly isolated Stenotrophomonas maltrophilia CCTCC M 204024. Appl Microbiol Biotechnol 71, 638–645 (2006). https://doi.org/10.1007/s00253-005-0201-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0201-9