Abstract
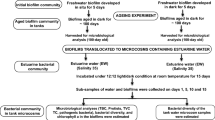
Ballast tank biofilms pose an additional risk of microbial invasion if sloughed off during ballasting operations, yet their significance and invasion biology is poorly understood. In this study, biofilms developed in marine and freshwater locations were exposed to prolonged darkness and aging by mimicking ballast water conditions in the laboratory. Upon prolonged darkness, the decay of phytoplankton, as indicated by the decrease in chlorophyll a in marine biofilms, led to remineralization and enhanced bacterial and protist populations. However, the same trend was not observed in the case of freshwater biofilms wherein the microbial parameters (i.e., bacteria, protists) and chlorophyll a decreased drastically. The bacterial community structure in such conditions was evaluated by real-time quantitative PCR (qPCR), and results showed that the biofilm bacterial communities changed significantly over a period of time. α-Proteobacteria was the most stable taxonomic group in the marine biofilms under dark conditions. However, β-proteobacteria dominated the freshwater biofilms and seemed to play an important role in organic matter remineralization. γ-Proteobacteria, which includes most of the pathogenic genera, were affected significantly and decreased in both the types of biofilms. This study revealed that marine biofilm communities were able to adapt better to the dark conditions while freshwater biofilm communities collapsed. Adaptation of tolerant bacterial communities, regeneration of nutrients via cell lysis, and presence of grazers appeared to be key factors for survival upon prolonged darkness. However, the fate of biofilm communities upon discharge in the new environment and their invasion potential is an important topic for future investigation.







Similar content being viewed by others
References
Tamelander J, Riddering L, Haag F, Matheickal J (2010) Guidelines for development of a National Ballast Water Management Strategy GEF-UNDP-IMO GloBallast, London, UK and IUCN, Gland, Switzerland GloBallast Monograph Series No. 18
Williams RJ, Griffiths FB, Van der Wal EJ, Kelly J (1988) Cargo vessel ballast water as a vector for the transport of non-indigenous marine species. Estuar Coast Shelf S 26:409–420
Ruiz GM, Rawlings TK, Dobbs FC, Drake LA, Mullady T, Huq A, Colwell RR (2000) Global spread of microorganisms by ships. Nature 408:49–50
Drake LA, Meyer AE, Forsberg RL, Baier RE, Doblin MA, Heinemann S, Johnson WP, Koch M, Rublee PA, Dobbs FC (2005) Potential invasion of microorganisms and pathogens via ‘interior hull fouling’: biofilms inside ballast water tanks. Biol Invasions 7:969–982
Mimura H, Katakura R, Ishida H (2005) Changes of microbial populations in a ship’s ballast water and sediments on a voyage from Japan to Qatar. Mar Pollut Bull 50:751–757
Drake LA, Doblin MA, Dobbs FC (2007) Potential microbial bioinvasions via ships’ ballast water, sediment, and biofilm. Mar Pollut Bull 55:333–341
Rivera IN, Souza KM, Souza CP, Lopes RM (2013) Free-living and plankton-associated vibrios: assessment in ballast water, harbor areas, and coastal ecosystems in Brazil. Front Microbiol 3:443. https://doi.org/10.3369/fmicb.2012.DD443
To KM, Jiamin D (2002) The environmental impacts of port and harbour activities: ballast water management (doctoral dissertation, the University of Hong Kong (Pokfulam, Hong Kong) pp. 1-79
Roszak DB, Grimes DJ, Colwell RR (1984) Viable but non recoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol 30:334–338
Hallegraeff GM, Bolch CJ (1992) Transport of diatom and dinoflagellate resting spores in ships ballast water: implications for plankton biography and aquaculture. J Plankton Res 14:1067–1084
Dickman MD, Zhang FZ (1999) Mid-ocean exchange of container vessel ballast water. 2: effects of vessel type in the transport of diatoms and dinoflagellates from Manzanillo, Mexico, to Hong Kong, China. Mar Ecol Prog Ser 176:253–262
Gollasch S, Lenz J, Dammer M, Andres HG (2000a) Survival of tropical ballast water organisms during a cruise from the Indian Ocean to the North Sea. J Plankton Res 22:923–937
Anil AC, Venkat K, Sawant SS, Dileepkumar M, Dhargalkar VK, Ramaiah N, Harkantra SN, Ansari ZA (2002) Marine bioinvasion: concern for ecology and shipping. Curr Sci 83:214–218
Carney KJ, Delany JE, Sawant SS, Mesbahi E (2011) The effects of prolonged darkness on temperate and tropical marine phytoplankton, and their implications for ballast water risk management. Mar Pollut Bull 62:1233–1244
Dobbs FC, Rogerson A (2005) Ridding ships’ ballast water of microorganisms. Environ Sci Technol 39:259A–264A
Emami K, Askari V, Ullrich M, Mohinudeen K, Anil AC, Khandeparker L, Burgess JG, Mesbahi E (2012) Characterization of bacteria in ballast water using MALDI-TOF mass spectrometry. PLoS One 7:e38515
Tomaru A, Kawachi M, Demura M, Fukuyo Y (2010) Denaturing gradient gel electrophoresis shows that bacterial communities change with mid-ocean ballast water exchange. Mar Pollut Bull 60:299–302
Khandeparker L, Anil AC (2013) Association of bacteria with marine invertebrates: implications for ballast water management. Ecosyst Health 10:268–276
Baier RE, Forsberg RL, Meyer AE, Lundquist DC (2014) Ballast tank biofilms resist water exchange but distribute dominant species. In Management of Biological Invasions Regional Euro-Asian Biological Invasions Centre (REABIC) 5:241–244
Hall-Stoodley L, Stoodley P (2005) Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol 13:7–10
Shikuma NJ, Hadfield MG (2010) Marine biofilms on submerged surfaces are a reservoir for Escherichia coli and Vibrio cholerae. Biofouling 26:39–46
Tomaru A, Kawachi M, Demura M, Fukuyo Y (2014) Changes in microbial communities, including both uncultured and culturable bacteria, with mid-ocean ballast-water exchange during a voyage from Japan to Australia. PLoS One 9:e96274
Hallegraeff GM, Bolch CJ (1991) Transport of toxic dinoflagellate cysts via ships’ ballast water. Mar Pollut Bull 22:27–30
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of nonindigenous marine organisms. Science 261:78–82
Rigby G, Hallegraeff GM (1993) Ballast water exchange trials and marine plankton distribution on the MV ‘Iron Whyalla’. Australian Government Publishing Service, Canberra 2:1–123
Rigby G, Hallegraeff GM (1994) The transfer and control of harmful marine organisms in shipping ballast water: behaviour of marine plankton and ballast water exchange trials on the MV ‘Iron Whyalla’. J Mar Environ Eng 1:91–110
Fukuyo Y, Ikegami T, Murase T (1995) Unwanted aquatic organisms in ballast tank. Report of the ballast water treatment by using main engine water cooling circuit and findings of the on board research. ICES, CM 1995/O 12:1–12
Gollasch S, Rosenthal H, Botnen H, Hamer J, Laing I, Leppäkoski E, Macdonald E, Minchin D, Nauke M, Olenin S, Utting S, Voigt M, Wallentinus I (2000b) Fluctuations of zooplankton taxa in ballast water during short-term and long-term ocean-going voyages. Int Rev Hydrobiol 85:597–608
Klein G, MacIntosh K, Kaczmarska I, Ehrman J (2010) Diatom survivorship in ballast water during trans-Pacific crossings. Biol Invasions 12:1031–1044
Villac MC, Kaczmarska I, Ehrman JM (2013) The diversity of diatom assemblages in ships, ballast sediments: colonization and propagule pressure on Canadian ports. J Plankton Res 35:1267–1282
Dagg M (1977) Some effects of patchy food environments on copepods. Limnol Oceanogr 22:99–107
Carlton JT (1985) Transoceanic and interoceanic dispersal of coastal marine organisms: the biology of ballast water. Oceanogr Mar Biol 23:313–374
Drake LA, Ruiz GM, Galil BS, Mullady TL, Friedmann DO, Dobbs FC (2002) Microbial ecology of ballast water during a transoceanic voyage and the effects of open-ocean exchange. Mar Ecol Prog Ser 233:13–20
Saccà A (2015) Invasive aquatic microorganisms: patterns of introduction and impacts. Biological invasions. Patterns, management and economic impacts: Nova Science Publishers, Inc. Vancouver 1–37
Bhosle NB, Garg A, Fernandes L, Citon P (2005) Dynamics of amino acids in the conditioning film developed on glass panels immersed in the surface seawaters of Dona Paula Bay. Biofouling 21:99–107
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, 173 pp
Pfeffer C, Oliver JD (2003) A comparison of thiosulphate citrate-bile salts-sucrose (TCBS) agar and thiosulphatechloride-iodide (TCI) agar for the isolation of Vibrio species from estuarine environments. Lett Appl Microbiol 36:150–151
Khandeparker L, Anil AC, Naik SD, Gaonkar CC (2015) Daily variations in pathogenic bacterial populations in a monsoon influenced tropical environment. Mar Pollut Bull 96:337–343
Khandeparker L, Eswaran R, Gardade L, Kuchi N, Mapari K, Naik SD, Anil AC (2017) Elucidation of the tidal influence on bacterial populations in a monsoon influenced estuary through simultaneous observations. Environ Monit Assess 189:41
Christaki U, Courties C, Massana R, Catala P, Lebaron P, Gasol JM, Zubkov MV (2011) Optimized routine flow cytometric enumeration of heterotrophic flagellates using SYBR Green I. Limnol Oceanogr Methods 9:329–339
Miller DN, Bryant JE, Madsen EL, Ghiorse WC (1999) Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl Environ Microbiol 65:4715–4724
De Gregoris TB, Aldred N, Clare AS, Burgess JG (2011) Improvement of phylum-and class specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods 86:351356
Ashelford KE, Weightman AJ, Fry JC (2002) PRIMROSE: a computer program for generating and estimating the phylogenetic range of 16S rRNA oligonucleotide probes and primers in conjunction with the RDP-II database. Nucleic Acids Res 30:3481–3489
ter Braak CJ, Verdonschot PF (1995) Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57:255–289
ter Braak CJ, Smilauer P (2002) CANOCO reference manual and Cano Draw for Windows user’s guide: software for canonical community ordination (version 4.5)
Leps J, Smilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University press
Tang KW, Freund CS, Schweitzer CL (2006) Occurrence of copepod carcasses in the lower Chesapeake Bay and their decomposition by ambient microbes. Estuar Coast Shelf Sci 68:499–508
Navarro E, Serrano-Heras G, Castaño MJ, Solera J (2015) Real-time PCR detection chemistry. Clin Chim Acta 439:231–250.
Methé BA, Hiorns WD, Zehr JP (1998) Contrasts between marine and freshwater bacterial community composition: analyses of communities in Lake George and six other Adirondack lakes. Limnol Oceanogr 43:368–374
Nold SC, Zwart G (1998) Patterns and governing forces in aquatic microbial communities. Aquat Ecol 32:17–35
Nogales B, Aguilό-Ferretjans MM, Martίn-Cardona C, Lalucat J, Bosch R (2007) Bacterial diversity, composition and dynamics in and around recreational coastal areas. Environ Microbiol 9:1913–1929
Neyland EB (2009) Bacteria in ballast water: the shipping industry’s contributions to the transport and distribution of microbial species in Texas. MSc dissertation. Texas A&M University
Williams KP, Sobral BW, Dickerman AW (2007) A robust species tree for the Alphaproteobacteria. J Bacteriol 189:4578–4586
Campagne S, Damberger FF, Kaczmarczyk A, Francez-Charlot A, Allain FHT, Vorholt JA (2012) Structural basis for sigma factor mimicry in the general stress response of Alphaproteobacteria. Proc Natl Acad Sci U S A 109:1405–1414
Parry JD (2004) Protozoan grazing of freshwater biofilms. Adv Appl Microbiol 54:167–196
Manz W, Wendt-Potthoff K, Neu TR, Szewzyk U, Lawrence JR (1999) Phylogenetic composition, spatial structure, and dynamics of lotic bacterial biofilms investigated by fluorescent in situ hybridization and confocal laser scanning microscopy. Microb Ecol 37:225237
Araya R, Tani K, Takagi T, Yamaguchi N, Nasu M (2003) Bacterial activity and community composition in stream water and biofilm from an urban river determined by fluorescent in situ hybridization and DGGE analysis. FEMS Microbiol Ecol 43:111–119
Edwards JL, Smith DL, Connolly J, McDonald JE, Cox MJ, Joint I, Edwards C, McCarthy AJ (2010) Identification of carbohydrate metabolism genes in the metagenome of a marine biofilm community shown to be dominated by Gammaproteobacteria and Bacteroidetes. Genes 1:371384
Walker DI, Keevil CW (2015) Low-concentration diffusible molecules affect the formation of biofilms by mixed marine communities. Cogent Biology 1:1103830
Munn C (2011) Marine microbiology: ecology and applications. Garland Science, London
Gao X, Olapade OA, Leff LG (2005) Comparison of benthic bacterial community composition in nine streams. Aquat Microb Ecol 40:51–60
Parveen B, Reveilliez JP, Mary I, Ravet V, Bronner G, Mangot JF, Domaizon I, Debroas D (2011) Diversity and dynamics of free-living and particle-associated Betaproteobacteria and Actinobacteria in relation to phytoplankton and zooplankton communities. FEMS Microbiol Ecol 77:461–476
Gasol JM, Comerma M, García JC, Armengol J, Casamayor EO, Kojecká P, Šimek K (2002) A transplant experiment to identify the factors controlling bacterial abundance, activity, production, and community composition in a eutrophic canyon-shaped reservoir. Limnol Oceanogr 47:62–77
Wu Q, Zwart G, Schauer M, Kamst-van Agterveld M, Hahn M (2006) Bacterioplankton community composition along a salinity gradient of sixteen high-mountain lakes located on the Tibetan Plateau, China. Appl Environ Microbiol 72:5478–5485
Rusch DB, Halpern AL, Sutton G, Heidelberg KB, Williamson S, Yooseph S, Wu D, Eisen JA, Hoffman JM, Remington K, Beeson K (2007) The Sorcerer II global ocean sampling expedition: northwest Atlantic through eastern tropical Pacific. PLoS Biol 5:77
Biers EJ, Sun S, Howard EC (2009) Prokaryotic genomes and diversity in surface ocean waters: interrogating the global ocean sampling metagenome. Appl Environ Microbiol 75:2221–2229
Ramette A, Tiedje JM (2007) Biogeography: an emerging cornerstone for understanding prokaryotic diversity, ecology, and evolution. Microb Ecol 53:197–207
Balzer M, Witt N, Flemming HC, Wingender J (2010) Faecal indicator bacteria in river biofilms. Water Sci Technol 61:1105
Colwell RR (2000) Viable but nonculturable bacteria: a survival strategy. J Infect Chemother 6:121–125
Colwell RR, Brayton PR, Grimes DU, Rosazk DB, Huq SA, Palmer LM (1985) Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Nat Biotechnol 3:817–820
Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S (2014) Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front Public Health
Matz C, Kjelleberg S (2005) Off the hook–how bacteria survive protozoan grazing. Trends Microbiol 13:302–307
Gerea M, Queimaliños C, Schiaffino MR, Izaguirre I, Forn I, Massana R, Unrein F (2013) In situ prey selection of mixotrophic and heterotrophic flagellates in Antarctic oligotrophic lakes: an analysis of the digestive vacuole content. J Plankton Res 35:201–212
Martinez-Garcia M, Brazel D, Poulton NJ, Swan BK, Gomez ML, Masland D, Sieracki ME, Stepanauskas R (2012) Unveiling in situ interactions between marine protists and bacteria through single cell sequencing. ISME J 6:703–707
Aguilera MA, Navarrete SA, Broitman BR (2013) Differential effects of grazer species on periphyton of a temperate rocky shore. Mar Ecol Prog Ser 484:63–78
Gude H (1989) The role of grazing on bacteria in plankton succession. In: Sommer U (ed) Plankton ecology. Succession in plankton communities. Springer Verlag, Berlin, pp 337–364
Arndt H, Schmidt-Denter K, Auer B, Weitere M (2003) Protozoans and biofilms. In fossil and recent biofilms (pp. 161-179). Springer Netherlands
Lee YM, Cho KH, Hwang K, Kim EH, Kim M, Hong SG, Lee HK (2016) Succession of bacterial community structure during the early stage of biofilm development in the Antarctic marine environment. Korean J Microbiol 52:49–58
Martiny JBH, Bohannan BJ, Brown JH, Colwell RK, Fuhrman JA, Green JL, Horner-Devine MC, Kane M, Krumins JA, Kuske CR, Morin PJ (2006) Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4:102–112
Langenheder S, Ragnarsson H (2007) The role of environmental and spatial factors for the composition of aquatic bacterial communities. Ecology 88:2154–2161
Litchman E (2010) Invisible invaders: non-pathogenic invasive microbes in aquatic and terrestrial ecosystems. Ecol Lett 13:1560–1572
Acknowledgements
We are grateful to the Director, National Institute of Oceanography, for his support and encouragement. We gratefully acknowledge Dr. A.C. Anil, Head of the department for his support and also thank colleagues of the BBD department for their help. This work was supported by Ballast Water Management Program, India (Ministry of Shipping and DG shipping) (GAP 2429) and CSIR funded Ocean Finder Program (PSC 0105). The first author (NH) would like to acknowledge the Jawaharlal Nehru Scholarship (JNS) India, for the research fellowship and is the registered Ph.D. student at the Department of Marine Sciences, Goa University. This is NIO contribution No 6157.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hede, N., Khandeparker, L. Influence of Darkness and Aging on Marine and Freshwater Biofilm Microbial Communities Using Microcosm Experiments. Microb Ecol 76, 314–327 (2018). https://doi.org/10.1007/s00248-018-1149-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-018-1149-5