Abstract
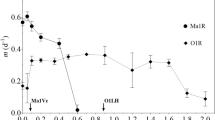
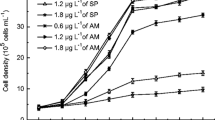
The cyanobacterium Microcystis aeruginosa is a mesophilic freshwater organism, which cannot tolerate sulphide. However, it was possible to isolate a sulphide-resistant (S r) mutant strain that was able to survive in a normally lethal medium sulphide. In order to evaluate the cost of the mutation conferring sulphide resistance in the S r strain of M. aeruginosa, the morphology and the photosynthetic performance were compared to that found in the wild-type, sulphide-sensitive (S s) strain. An increase in size and a disrupted morphology was observed in S r cells in comparison to the S s counterpart. Phycoerythrin and phycocyanin levels were higher in the S r than in the S s cells, whereas a higher carotenoid content, per unit volume, was found in the S s strain. The irradiance-saturated photosynthetic oxygen-production rate (GPR max) and the photosynthetic efficiency (measured both by oxygen production and fluorescence, α GPR and α ETR) were lower in the S r strain than in the wild-type. These results appear to be the result of package effect. On the other hand, the S r strain showed higher quantum yield of non-photochemical quenching, especially those regulated mechanisms (estimated throughout q N and Y(NPQ)) and a significantly lower slope in the maximum quantum yield of light-adapted samples (F v ′/F m ′) compared to the S s strain. These findings point to a change in the regulation of the quenching of the transition states (q T ) in the S r strain which may be generated by a change in the distribution of thylakoidal membranes, which somehow could protect metalloenzymes of the electron transport chain from the lethal effect of sulphide.







Similar content being viewed by others
Abbreviations
- a*:
-
Chlorophyll-specific optical absorption cross section
- Chla :
-
Chlorophyll a
- E :
-
Incident irradiance
- E 0.5 :
-
Half-saturation irradiance
- ETR :
-
Electron transport rate computed as Y(II) × E × a* × 0.5
- F 0 :
-
Minimal fluorescence of dark-adapted cells
- F 0′:
-
Minimal fluorescence of illuminated cells
- F t :
-
Steady-state fluorescence
- F m :
-
Maximal fluorescence of dark-adapted cells
- F m ′:
-
Maximal fluorescence of illuminated cells
- F v :
-
Variable fluorescence of dark-adapted cells computed as F m − F 0
- F v ′:
-
Variable fluorescence of illuminated cells computed as F m ′ − F 0′
- F v /F m :
-
Maximum quantum yield of dark-adapted cells computed as (F m − F 0) / F m
- F v ′/F m ′:
-
Maximum quantum yield of illuminated cells computed as (F m ′ − F 0′) / F m ′
- GPR :
-
Gross photosynthetic oxygen-production rate
- GPR max :
-
Irradiance-saturated GPR
- NPQ :
-
Non-photochemical quenching parameter computed as Y(NPQ) / Y(NO)
- PC:
-
Phycocyanin
- PE:
-
Phycoerythrin
- q E :
-
Energy-dependent quenching coefficient
- q I :
-
Photoinhibitory quenching coefficient
- q N :
-
Non-photochemical quenching coefficient computed as 1 − [(F m ′ − F 0′) / (F m − F 0)]
- q P :
-
Photochemical quenching coefficient computed as (F m ′ − F t ) / (F m ′ − F 0′)
- q T :
-
Quenching associated with “state 1–state 2” transition
- S r :
-
Sulphide-resistant (strain)
- S s :
-
Sulphide-sensitive (strain)
- TC:
-
Total carotenoids
- Y(II):
-
Photochemical efficiency of PSII computed as (F m′ - F t)/F m′
- Y(NO):
-
Quantum yield of non-regulated non-photochemical quenching computed as F t / F m
- Y(NPQ):
-
Quantum yield of regulated non-photochemical quenching computed as (F t / F m ′) − (F t / F m )
- α ETR :
-
Photosynthetic efficiency calculated from ETR-E relationship
- α GPR :
-
Photosynthetic efficiency calculated from GPR-E relationship
References
Li C, Love GD, Lyons TW, Fike DA, Sessions AL, Chu X (2010) A stratified redox model for the Ediacaran ocean. Science 328:80–83
Grice K, Cao C, Love GD, Böttcher ME, Twitchett RJ, Grosjean E, Summons RE, Turgeon SC, Dunning W, Jin Y (2005) Photic zone euxinia during the Permian-Trassic superanoxic event. Science 307:706–709
Mathai JC, Missner A, Kügler P, Saparov SM, Zeidel ML, Lee JK, Pohl P (2009) No facilitator required for membrane transport of hydrogen sulphide. Proc Natl Acad Sci U S A 106:16633–16638
Bagarinao T (1992) Sulphide as an environmental factor and toxicant: tolerance and adaptations in aquatic organisms. Aquat Toxicol 24:21–62
Stal LJ (1995) Physiological ecology of cyanobacteria in microbial mats and other communities. New Phytol 131:1–32
Cohen Y, Jørgensen BB, Revsbech NP, Poplawski R (1986) Adaptation to hydrogen sulphide of oxygenic and anoxygenic photosynthesis among cyanobacteria. Appl Environ Microbiol 51:398–407
Miller SR, Bebout BM (2004) Variation in sulphide tolerance of photosystem II in phylogenetically diverse cyanobacteria from sulfidic habitats. Appl Environ Microbiol 70:736–744
Castenholz RW (1977) The effect of sulphide on the blue-green algae of hot springs. II. Yellowstone National Park. Microb Ecol 3:79–105
Dodds WK, Castenholz RW (1990) Sulfide and pH effects on variable fluorescence of photosystem II in two strains of the cyanobacterium Oscillatoria amphigranulata. Photosynth Res 24:265–271
Cohen Y, Jørgensen BB, Padan E, Shilo M (1975) Sulfide-dependent anoxygenic photosynthesis in the cyanobacterium Oscillatoria limnetica. Nature 257:489–491
Czyzewski BK, Wang DN (2012) Identification and characterization of a bacterial hydrosulphide ion channel. Nature 483:494–497
Fernández-Arjona M, Bañares-España E, García-Sanchez MJ, Hernández-Lopez M, López-Rodas V, Costas E, Flores-Moya A (2013) Disentangling mechanisms involved in the adaptation of photosynthetic microorganisms to the extreme sulphureous water from Los Baños de Vilo (S Spain). Microb Ecol 66:742–751
Cooper S (1991) Bacterial growth and division. Biochemistry and regulation of prokaryotic and eukaryotic division cells. Academic, San Diego
Wellburn AR (1994) The spectral determinations of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Beer S, Eshel A (1985) Determining phycoerithrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust J Mar Freshwat Res 36:785–792
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4: 1–9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Schreiber U (1986) Detection of rapid induction kinetics with a new type of high-frecuency modulater chlorophyll fluorometer. Photosynth Res 9:261–272
Maxwell K, Johnson GN (2000) Chrorophyll fluorescence - a practical guide. J Exp Bot 51:659–668
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Dubinsky Z, Falkowski PG, Post AF, Hes U (1986) A system for measuring phytoplankton photosynthesis in a defined light field with an oxygen electrode. J Plankton Res 9:607–612
Morris EP, Kromkamp JC (2003) Influence of temperature on the relationship between oxygen- and fluorescence-based estimates of photosynthetic parameters in marine benthic diatom (Cylindrotheca closterium). Eur J Phycol 38:133–142
van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components- calculation of qP and Fv′/Fm′ without measuring F 0 ′. Photosynth Res 54:135–142
Klughammer C, Schreiber U (2008) Complementary PSII quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl Notes 1:27–35
Beardall J, Allen D, Bragg J, Finkel ZV, Flynn KJ, Quigg A, Alwyn T, Rees V, Richardson A, Raven JA (2009) Allometry and stoichiometry of unicellular, colonial and multicellular phytoplankton. New Phytol 181:295–309
Denny M (1992) Air and water. The biology and physics of life’s media. Princeton University Press, Princeton
Raven JA (1998) Small is beautiful. The picophytoplankton. Funct Ecol 98:503–513
Costas E, López-Rodas V (2006) Copper sulphate and DCMU-herbicide treatments increase asymmetry between sister cells in the toxic cianobacteria Microcystis aeruginosa: implications for detecting environmental stress. Water Res 40:2447–2451
López Rodas V, Flores-Moya A, Maneiro E, Perdigones N, Marvá F, García ME, Costas E (2006) Resistance to glyphosate in the cyanobacterium Microcystis aeruginosa as result of preselective mutations. Evol Ecol 21:535–547
Agustí S (1991) Allometric scaling of light absorption and scattering by phytoplankton cells. Can J Fish Aquat Sci 48:763–767
Zhu Y, Graham JE, Ludwing M, Xiong W, Alvey RM, Shen G, Bryant DA (2010) Roles of xanthophyll carotenoids in protection against photoinhibition and oxidative stress in the cyanobacterium Synechococcus sp. strain PCC 7002. Arch Biochem Biophys 504:86–99
Jahns P, Holzwarth AR (2012) The role of the xanthophylls cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Kirilovsky D, Kerfeld CA (2012) The orange carotenoid protein in photoprotection of photosystem II in cyanobacteria. Biochim Biophys Acta 1817:158–166
Joshua S, Mullineaux CW (2004) Phycobilisome diffusion is required for light-state transitions in cyanobacteria. Plant Physiol 135:2112–2119
Mullineaux CW, Allen JF (1990) State 1-State 2 transitions in the cyanobacterium Synechococcus 6301 are controlled by the redox state of electron carriers between Photosystems I and II. Photosynth Res 23:297–311
Mullineaux CW (2014) Electron transport and light-harvesting switches in cyanobacteria. Front Plant Sci 5:1–6
Sarcina M, Tobin MJ, Mullineaux CW (2001) Diffusion of phycobilisomes on the thylakoid membranes of cyanobacterium Synechococcus 7942. Effects of phycobilisome size, temperature, and membrane lipid composition. J Biol Chem 276:46830–46834
Bañares-España E, Kromkamp JC, López-Rodas V, Costas E, Flores-Moya A (2013) Photoacclimation of cultured strains of the cyanobacterium Microcystis aeruginosa to high-light and low-light conditions. FEMS Microbiol Ecol 83:700–710
Flameling IA, Kromkamp J (1998) Light dependence of quantum yields for PSII charge separation and oxygen evolution in eukaryotic algae. Limnol Oceanogr 43:284–297
Zorz JK, Allanach JR, Murphy CD, Roodvoets MS, Campbell DA, Cockshutt AM (2015) The RUBISCO to Photosystem II Ratio Limits the Maximum Photosynthetic Rate in Picocyanobacteria. Life 5:403–417
Grzymski J, Johnsen G, Sakshaug E (1997) The significance of intracellular self-shading on the Biooptical properties of Brown, Red and Green macroalgae. J Phycol 33:408–414
Huot Y, Babin M (2010) Overview of fluorescence protocols: theory, basic concepts, and practice. In: Suggett DJ, Prásil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic science: methods and applications, developments in applied phycology, vol 4. Springer, Berlin, pp 31–74
Campbell D, Hurry V, Clarke AK, Gustaffson P, Öquist G (1998) Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev 62:667–683
Simis SGH, Huot Y, Babin M, Seppälä ML (2012) Optimization of variable fluorescence measurements of phytoplankton communities with cyanobacteria. Photosynth Res 112:13–30
Campbell D, Zhou G, Gustafsson P, Öquist G, Clarke K (1995) Electron transport regulates exchange of two forms of Photosystem II D1 protein in the cyanobacterium Synechococcus. EMBO J 14:5457–5466
Miller AG, Espie GS, Canvin DT (1990) Active transport of inorganic carbon increases the rate of O2 photoreduction by the cyanobacterium Synechococcus UTEX 625. Plant Physiol 88:6–9
Vermaas WFJ, Shen G, Styring S (1994) Electrons generated by photosystem II are utilized by an oxidase in the absence of photosystem I in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett 337:103–108
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basis. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125:1558–1566
Mullineaux CW (2008) Phycobilisome-reaction centre interaction in cyanobacteria. Photosynth Res 95:175–182
Bañares-España E, López-Rodas V, Salgado C, Costas E, Flores-Moya A (2006) Interstrain variability in the photosynthetic use of inorganic carbon, exemplified by the pH compensation point, in the cyanobacterium Microcystis aeruginosa. Aquat Bot 85:159–162
Acknowledgments
This work was financially supported by the projects UMA-FEDER 2014–15 (FC14-CGL-08) and Ministerio de Economía y Competitividad (CGL2014-53862-P). The acquisition of the FlowCAM by the University of Málaga was co-financed by the 2008–2011 FEDER programme for Scientific-Technique Infrastructure (UNMA08-1E005). We thank Dr. Douglas Campbell and an anonymous reviewer for their suggestions to improve the manuscript. Dr. Eric C. Henry kindly revised the English style and usage.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bañares-España, E., del Mar Fernández-Arjona, M., García-Sánchez, M.J. et al. Sulphide Resistance in the Cyanobacterium Microcystis aeruginosa: a Comparative Study of Morphology and Photosynthetic Performance Between the Sulphide-Resistant Mutant and the Wild-Type Strain. Microb Ecol 71, 860–872 (2016). https://doi.org/10.1007/s00248-015-0715-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-015-0715-3