Abstract
Background
Given the recent concerns about gadolinium-based contrast agent safety, dose reduction strategies are being investigated.
Objective
To compare half-dose and standard full-dose gadoterate meglumine at 3-tesla (T) MRI in paediatric bone and soft-tissue diseases.
Materials and methods
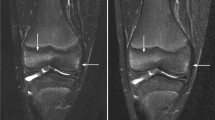
We prospectively enrolled 45 children (age range 2.7 months to 17.5 years, median age 8.7 years, 49 total anatomical segments) with bone and soft-tissue diseases (neoplastic, inflammatory/infectious, ischaemic and vascular) imaged at 3-T MRI. Two consecutive half-doses of gadoterate meglumine (0.05 mmol/kg body weight) were administered. Two sets of post-contrast T1-weighted images were obtained, one after the first half dose and the other after the second half dose. For qualitative analysis, three radiologists, masked to the gadolinium dose, compared the diagnostic quality of the images. For quantitative analysis, we compared signal-to-noise ratio and contrast-to-noise ratio at half and full doses.
Results
Signal-to-noise ratio and contrast-to-noise ratio did not vary significantly between the two groups. Qualitative analysis yielded excellent image quality in both post-contrast image datasets (Cohen κ=0.8).
Conclusion
In paediatric bone and soft-tissue 3-T MRI, it is feasible to halve the standard dose of gadoterate meglumine without losing image quality.








Similar content being viewed by others
References
Marckmann P, Skov L, Rossen K et al (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17:2359–2362
Nardone B, Saddleton E, Laumann AE et al (2014) Pediatric nephrogenic systemic fibrosis is rarely reported: a RADAR report. Pediatr Radiol 44:173–180
American College of Radiology Committee on Drugs and Contrast Media (2017) ACR manual on contrast media version 10.3. American College of Radiology, Reston
Kanda T, Ishii K, Kawaguchi H et al (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Bae S, Lee HJ, Han K et al (2017) Gadolinium deposition in the brain: association with various GBCAs using a generalized additive model. Eur Radiol 27:3353–3361
Fraum TJ, Ludwig DR, Bashir MR, Fowler KJ (2017) Gadolinium-based contrast agents: a comprehensive risk assessment. J Magn Reson Imaging 46:338–353
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
Gale EM, Caravan P, Rao AG et al (2017) Gadolinium-based contrast agents in pediatric magnetic resonance imaging. Pediatr Radiol 47:507–521
Scala M, Koob M, de Buttet S et al (2017) A pharmacokinetics, efficacy, and safety study of gadoterate meglumine in pediatric subjects aged younger than 2 years. Invest Radiol 53:70–79
Dehkharghani S, Qiu D, Albin LS, Saindane AM (2015) Dose reduction in contrast-enhanced cervical MR angiography: field strength dependency of vascular signal intensity, contrast administration, and arteriographic quality. AJR Am J Roentgenol 204:W701–W706
Crisi G, Filice S, Erb G, Bozzetti F (2016) Effectiveness of a high relaxivity contrast agent administered at half dose in dynamic susceptibility contrast MRI of brain gliomas. J Magn Reson Imaging 45:500–506
Schueller-Weidekamm C, Lodemann KP, Grisar J et al (2013) Contrast-enhanced MR imaging of hand and finger joints in patients with early rheumatoid arthritis: do we really need a full dose of gadobenate dimeglumine for assessing synovial enhancement at 3T? Radiology 268:161–169
Waugh SA, Ramkumar PG, Gandy SJ et al (2009) Optimization of the contrast dose and injection rates in whole-body MR angiography at 3.0T. J Magn Reson Imaging 30:1059–1067
Sherry AD, Caravan P, Lenkinski RE (2009) Primer on gadolinium chemistry. J Magn Reson Imaging 30:1240–1248
McDonald RJ, McDonald JS, Kallmes DF et al (2017) Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology 27:161595
Robert P, Violas X, Grand S et al (2016) Linear gadolinium-based contrast agents are associated with brain gadolinium retention in healthy rats. Invest Radiol 51:73–82
Roberts DR, Chatterjee AR, Yazdani M et al (2016) Pediatric patients demonstrate progressive T1-weighted hyperintensity in the dentate nucleus following multiple doses of gadolinium-based contrast agent. AJNR Am J Neuroradiol 37:2340–2347
Flood TF, Stence NV, Maloney JA, Mirsky DM (2017) Pediatric brain: repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology 282:222–228
Rabruch A, Haase R, Kickingereder P (2017) Pediatric brain: no increased signal intensity in the dentate nucleus on unenhanced T1-weighted MR images after consecutive exposure to a macrocyclic gadolinium-based contrast agent. Radiology 283:828–836
Tibussek D, Rademacher C, Caspers J et al (2017) Gadolinium brain deposition after macrocyclic gadolinium administration: a pediatric case-control study. Radiology 21:161151
Rossi Espagnet MC, Bernardi B, Pasquini L et al (2017) Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol 47:1345–1352
Maximova N, Gregori M, Zennaro F et al (2016) Hepatic gadolinium deposition and reversibility after contrast agent-enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology 281:418–426
Kanda T, Nakai Y, Hagiwara A et al (2017) Distribution and chemical forms of gadolinium in the brain: a review. Br J Radiol 27:20170115
Frenzel T, Apte C, Jost G et al (2017) Quantification and assessment of the chemical form of residual gadolinium in the brain after repeated administration of gadolinium-based contrast agents: comparative study in rats. Invest Radiol 52:396–404
European Medicines Agency (2017) EMA’s final opinion confirms restrictions on use of linear gadolinium agents in body scans: recommendations conclude EMA’s scientific review of gadolinium deposition in brain and other tissues. Report EMA/457616/2017. European Medicines Agency, London
Soyer P, Dohan A, Patkar D, Gottschalk A (2016) Observational study on the safety profile of gadoterate meglumine in 35,499 patients: the SECURE study. J Magn Reson Imaging 45:988–997
Khan R (2016) MRI contrast agents: evolution of clinical practice and dose optimization. Top Magn Reson Imaging 25:157–161
Beiderwellen K, Kraff O, Laader A et al (2017) Contrast enhanced renal MR angiography at 7 tesla: how much gadolinium do we need? Eur J Radiol 86:76–82
Song KD, Kim SH, Lee J et al (2015) Half-dose gadoxetic acid-enhanced liver magnetic resonance imaging in patients at risk for nephrogenic systemic fibrosis. Eur J Radiol 84:378–383
Morelli JN, Gerdes CM, Zhang W et al (2013) Enhancement in a brain glioma model: a comparison of half-dose gadobenate dimeglumine versus full-dose gadopentetate dimeglumine at 1.5 and 3 T. J Magn Reson Imaging 38:306–311
Nael K, Meshksar A, Ellingson B et al (2014) Combined low-dose contrast-enhanced MR angiography and perfusion for acute ischemic stroke at 3T: a more efficient stroke protocol. AJNR Am J Neuroradiol 35:1078–1084
Sandhu GS, Rezaee RP, Jesberger J et al (2012) Time-resolved MR angiography of the legs at 3 T using a low dose of gadolinium: initial experience and contrast dynamics. AJR Am J Roentgenol 198:686–691
Kuo R, Panchal M, Tanenbaum L, Crues JV III (2007) 3.0 tesla imaging of the musculoskeletal system. J Magn Reson Imaging 25:245–261
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med 22:276–282
Meyer JS, Jaramillo D (2008) Musculoskeletal MR imaging at 3 T. Magn Reson Imaging Clin N Am 16:533–545
Vaneckova M, Herman M, Smith MP et al (2015) The benefits of high relaxivity for brain tumor imaging: results of a multicenter intraindividual crossover comparison of gadobenate dimeglumine with gadoterate meglumine (the BENEFIT study). AJNR Am J Neuroradiol 36:1589–1598
Runge VM (2017) Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA's Pharmacovigilance and Risk Assessment Committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol 52:317–323
Costelloe MC, Murphy AW Jr, Haygood TM et al (2011) Comparison of half-dose and full-dose gadolinium MR contrast on the enhancement of bone and soft tissue tumors. Skeletal Radiol 40:327–333
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Colafati, G.S., Rossi, E., Carducci, C. et al. Half-dose versus full-dose macrocyclic gadolinium at 3-T magnetic resonance imaging in paediatric bone and soft-tissue disease. Pediatr Radiol 48, 1724–1735 (2018). https://doi.org/10.1007/s00247-018-4204-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4204-y