Abstract
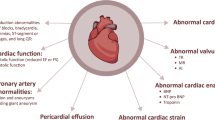
Multi-system inflammatory syndrome in children (MIS-C) causes widespread inflammation including a pancarditis in the weeks following a COVID infection. As we prepare for further coronavirus surges, understanding the medium-term cardiac impacts of this condition is important for allocating healthcare resources. A retrospective single-center study of 67 consecutive patients with MIS-C was performed evaluating echocardiographic and electrocardiographic (ECG) findings to determine the point of worst cardiac dysfunction during the admission, then at intervals of 6–8 weeks and 6–8 months. Worst cardiac function occurred 6.8 ± 2.4 days after the onset of fever with mean 3D left ventricle (LV) ejection fraction (EF) 50.5 ± 9.8%. A pancarditis was typically present: 46.3% had cardiac impairment; 31.3% had pericardial effusion; 26.8% demonstrated moderate (or worse) valvar regurgitation; and 26.8% had coronary dilatation. Cardiac function normalized in all patients by 6–8 weeks (mean 3D LV EF 61.3 ± 4.4%, p < 0.001 compared to presentation). Coronary dilatation resolved in all but one patient who initially developed large aneurysms at presentation, which persisted 6 months later. ECG changes predominantly featured T-wave changes resolving at follow-up. Adverse events included need for ECMO (n = 2), death as an ECMO-related complication (n = 1), LV thrombus formation (n = 1), and subendocardial infarction (n = 1). MIS-C causes a pancarditis. In the majority, discharge from long-term follow-up can be considered as full cardiac recovery is expected by 8 weeks. The exception includes patients with medium sized aneurysms or greater as these may persist and require on-going surveillance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In April 2020 following the first peak of the Covid 19 pandemic a new condition emerged in young people causing multi-system inflammation [1]. The clinical features included fever, rash, conjunctival injection, and gastrointestinal symptoms, occasionally progressing to multi-organ failure requiring intensive care support [2, 3] It has been named multisystem inflammatory syndrome in children (MIS-C) [4] or pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) [5]. This condition is induced by a cytokine storm [1, 6, 7], and although it overlaps with other inflammatory conditions, such as Kawasaki disease, it is thought to represent a new disease entity [8]. The effects on the heart are more widespread and severe than with acute covid myocarditis [9] with cardiac impairment (34%), [10] coronary artery involvement (13%) [11], and electrocardiogram (ECG) changes (67%) all described [12, 13].
The presence and severity of widespread cardiac involvement in this new condition highlights the importance of a detailed understanding of the cardiac course to foresee what future complications may arise. Most countries across the world now face the impact of on-going further surges of COVID and combined with the added and unknown effects of viral mutations and large numbers of unvaccinated young people, multiple waves of post-COVID pediatric multi-system inflammation patients are expected. This study presents the evolution of the cardiac manifestations through the acute phase to the 6 months follow-up of the first group of patients presenting to our institution. We analyze advanced echocardiographic functional indices and ECG changes to provide a comprehensive understanding of the longer lasting impact of this condition.
Methods
A retrospective single institution longitudinal study with sequential analysis of the cardiac manifestations in consecutive patients presenting to the Evelina London Children’s Hospital (ELCH) with features of MIS-C [4]. Clinical databases, patient records, and discharge summaries were used to identify patients diagnosed with MIS-C with at least 6 months of follow-up since the initial diagnosis was made. Local clinical databases were interrogated to assess patient demographics, blood results, treatment received, inotropic support, echocardiographic and ECG findings. All decisions regarding immune therapy were made by a dedicated multi-disciplinary team comprised of specialists in pediatric rheumatology, pediatric infectious diseases, general pediatrics and pediatric cardiology. Data were collected in accordance with the 1964 Helsinki declaration. Institutional ethics approval was obtained permitting retrospective review and use of echocardiographic data for clinical research and publication (09/H0802/116) without the need for informed written consent for publication.
Clinical and Laboratory Evaluation
Demographic data and SARS-CoV-2 case investigations were collected from healthcare records. Vital signs and cardiac biomarkers including Troponin-T and NT-proBNP were collected prospectively as part of routine clinical care.
Cardiac Imaging Evaluation Protocol
All patients had echocardiographic evaluation on initial admission. Echocardiograms and electrocardiograms (ECGs) were performed daily during the acute phase of the disease, at 6–8 weeks and; approximately 6 months after discharge. We defined three time-points for analysis: (1) worst echocardiogram during admission based on Global Longitudinal Strain (GLS) as a sensitive marker for dysfunction; (2) follow-up at 6–8 weeks; and (3) follow-up at 6–8 months. ECG data were also analyzed at the same timepoints.
Echocardiography
Philips IE33 and EPIQ 7C echocardiography systems (Philips Medical Systems, Andover, Massachusetts) were used to obtain imaging with offline processing using Philips IntelliSpace Cardiovascular (ISCV) and analysis using QLAB, Version 10.8.5 (Philips Medical Systems).
The echocardiograms included extensive functional assessment. Left ventricular (LV) function was quantified in accordance with published guidelines [14] and Z scores were calculated where appropriate [15]. 3D echocardiographic ejection fraction values were referenced according to Bernard et al [16].
Peak pulse wave tissue doppler indices (PW-TDI) measuring systolic and diastolic velocities of the basal septal and lateral segments of the LV were analyzed according to published standards [17]. The LV diastolic function and LV filling pressures were assessed using the mean of lateral and septal E/e′ values [18]. The four chamber, three chamber, two chamber views were used for 2D based speckle-tracking myocardial deformation echocardiographic analysis [19]. 3D echocardiography was used to calculate LV volumes and ejection fraction (EF) [20]. The pericardial effusion was graded according to the size including trivial (seen only in systole), mild (< 10 mm), moderate (10–20 mm), and severe (> 20 mm) in the parasternal long axis view [21].
The dimensions of the coronary arteries were expressed as z scores in accordance with the published reference standard [22]. Coronary artery dilation was defined by the presence of a Z score > 2 in the affected segment. All echocardiographic measurements and reports were reviewed prospectively at a daily multi-disciplinary meeting by attending consultants with expertise in echocardiography (PT, JW, KP, and OM).
Electrocardiography (ECGs)
All ECGs were recorded at standard 25 mm/s paper speed and gain setting of 10 mm/mV. The ECGs were scanned in high definition (400 dpi) and Adobe Photoshop (Adobe Systems) was used with a calibrated digital caliper tool, to measure ECG intervals (PR, QRSd, RR, QTc [3 consecutive QT intervals in leads II and V5, with preceding RR interval, corrected using the Bazett formula]) and amplitudes (R- and S-wave amplitudes in V1, V4, and V6 and T-wave amplitudes in leads I and V6). Computer-generated measurements for heart rate, QRS duration, and QRS axis were used and visually checked for accuracy (WR, LB).
Statistical Analysis
Statistical analysis was performed using SPSS v26. Variables were tested for normality using the Kolmogorov–Smirnov test. Repeated Measures Analysis of Variance (RM ANOVA) was performed to evaluate changes of all echocardiographic parameters assessed during hospitalization at 6 weeks and 6 months post discharge. The results for variables with normal distribution were reported as mean ± standard deviation, while the not normally distributed parameters were reported as median ± range. Statistical significance was defined as a two-tailed p-value of < 0.05.
Results
Demographics
Sixty seven patients were included in the study (the demographics are summarized in Table 1). Patients presented in a 7 weeks period between the 14th April 2020 and 4th June 2020. The mean age was 8.9 ± 4.6 years (range 4 months–16 years). Forty five patients (67.2%) were male and 22 (23.8%) were female. Only 3 patients (4.5%) had positive SARS-CoV-2 PCR from oropharyngeal swab sampling at presentation. Nineteen patients (28.4%) had negative antibody tests while 46 (68.6%) patients tested positive for either IgM or IgG antibodies for COVID-19. Two patients (3.0%) were not tested due to parental choice. Both children had a recent history of an infection consistent with SARS-CoV2 and their symptoms were consistent with a diagnosis of MIS-C hence they were still included in the case series. All patients received immunomodulation which was determined by the multi-disciplinary team. Table 2 shows the treatment received. Need for mechanical ventilation was required in 27.9% for mean duration of 3.3 ± 2.8 days. Inotropic support was required in 44% and 94% received some form of immuno-modulation.
Acute Phase Echocardiography Findings
The worst echocardiographic cardiac function defined by GLS was identified 6.6 ± 2.4 days after the onset of the febrile illness. Twenty-seven (40.3%) patients demonstrated impaired LV systolic performance, as assessed by M-mode-derived LV fractional shortening (FS). The 3D echocardiography assessment showed that 33 (49.2%) patients had reduced LV systolic function (3D LV EF < 55%). 2D strain analysis revealed 31 (46.3%) patients had abnormal LV global longitudinal strain (LV GLS greater than −16%) with a mean observed LV GLS of −15.5 ± 5.9% [23]. Eighteen patients (26.8%) had evidence of moderate valvar regurgitation or worse. Nine (13.4%) of these patients presented with moderate-to-severe mitral regurgitation and 8 (11.9%) of these patients presented with moderate-to-severe tricuspid regurgitation. Twenty-one (31.3%) patients were found to have a pericardial effusion—60% of which was graded as mild and 40% as moderate. None of the patients required effusion drainage.
Serial Echocardiographic Assessments
All patients underwent serial echocardiographic assessments during the initial admission, at 6–8 weeks (mean 59 ± 14 days since onset of illness) and then at 6–8 months (mean 159 ± 23 days since onset of illness) after discharge. This is shown in Table 3. There was a significant improvement of the LV systolic performance consistent across all the echocardiographic indices utilized to assess the LV systolic function observed at 6–8 weeks after discharge. There was no further improvement noted at the 6–8 months timepoint. The mean M-mode-derived FS was 30.9 ± 8.1% during the acute phase and improved to 37.5 ± 7.0%, p < 0.001 at the first follow-up appointment with no further improvement (p = 0.15 vs. first timepoint) at 6–8 months post discharge. The mean 3D LVEF during admission was 50.5 ± 9.8%, and recovered to 61.3 ± 4.4%, p < 0.001 at the time of the first follow-up appointment and was found to be similar (p = 0.517 vs. first timepoint) at 6–8 months see Fig. 1. Similarly, the initial mean LV GLS was −15.4 ± 4.9% during acute phase with a significant improvement (−19.1 ± 2.0%, p < 0.001) at 6–8 weeks post discharge which was sustained (p = 0.223 vs. first timepoint) at the time of the final follow-up assessment (Fig. 1a–c). The LV diastolic function assessed by mean E/e′ ratio improved at the first follow-up appointment compared to acute phase (median 6.74, range 3.74–16.50 vs median 7.40, range 4.45–15.10, p = 0.007) and remained unchanged at the time of the final follow-up appointment (Table 3). Importantly at the 6–8 weeks timepoint all patients had recovered their cardiac function with normal 3D LV EF values greater than 50% [16].
a Three dimensional Ejection Fraction (3D LVEF) improved at 6–8 weeks follow-up and remained in normal limits 6 months after. b Left ventricular Global Longitudinal Strain (GLS) was the first parameter to be affected in the course of MIS-C. It normalized at 6–8 weeks and remained within normal limits at 6 m follow-up. c The Left Ventricular Fractional Shortening was the least affected parameter in the LV function assessment and was not proven sensitive enough to assess cardiac function and the changes of it. However, there was improvement in the parameter values at 6–8 weeks follow-up and no significant further improvement at 6 months
Coronary Arteries
Eighteen (26.8%) patients had some degree of coronary dilatation during the admission echo based on a Z score > 2. On admission 7 patients (10.4%) were identified to have left main stem coronary artery (LMCA) dilatation (Z score > 2), 11 patients (16.4%) had left anterior descending (LAD) dilatation, and 3 patients (4.4%) left circumflex (LCx) dilatation. The right coronary artery (RCA) was found to be dilated (Z score > 2) in 9 patients (13.4%) in the acute phase see Table 3. Seven patients did not have their left circumflex coronary artery adequately visualized on their initial scan, these patients had no other coronary artery dilatation present. The circumflex was subsequently well seen on follow-up imaging and normal in size.
Serial echocardiographic assessments showed a significant reduction in Z scores for the LMCA (median + 0.15, range −2.98to+ 4.85 vs median −0.22, range −2.65 to + 3.86, p = 0.004) and LAD (median + 0.45 range −2.1 to + 10.00 vs median −0.15, range −2.51 to + 12.10, p = 0.02) at the 6–8 weeks assessment with further non-statistically significant reduction at the time of the final follow-up appointment. Similarly the Z score of the RCA in the acute phase (median −0.1, range −3.29 to + 4.20) significantly reduced by the time of the first follow-up appointment (median −0.60, range −2.7 to + 4.03, p < 0.001) without any further significant reduction at 6–8 months (Fig. 2).
Changes in coronary artery Z score measurements over time. Top left panel shows the right coronary artery (RCA). Top right panel shows the left main coronary artery (LMCA). Bottom left shows the left anterior descending artery (LAD). Bottom right shows the left circumflex artery (LCx). Timepoint one coincides with largest measurement during admission; timepoint two coincides with the first follow-up appointment at 6–8 weeks; and timepoint three coincides with the follow-up appointment at 6–8 months
Serial Electrocardiographic Assessments
A total of 53 children had suitable serial ECG data for all three review points. There were significant changes in T-waves (amplitude and polarity in chest leads) between the acute phase and first follow-up, without further changes into final follow-up. The percentage of children found to have lateral precordial T-wave inversion (in V4–V6) was 15% in acute phase, falling to 1.6% by the first follow-up, and 0% by the final follow-up. Flattened T-waves in V6 during the acute phase measuring 0.24 mV (−0.034 to 0.81 mV) showed a significant increase in amplitude by the first follow-up 0.44 mV (0.14 to 0.72 mV), p < 0.001, but then decreased on final follow-up by a lesser degree to 0.41 (0 to 0.93 mV), p = 0.003. As a result of a similar fluctuation in R-wave amplitude, a more consistent measure over time was T/R ratio in V6 showing a significant increase from the acute phase 0.18 (0 to 0.62) to first follow-up 0.28 (0.09 to 0.51), p < 0.01, which plateaued by final follow-up 0.29 (0.14–0.53), p = 0.34.
Other ECG parameters (heart rate, PR interval, QRS duration, and QTc), showed more variability over time, falling within normal ranges for age and any minor variations showed no further improvement over longer follow-up, as illustrated by the PR interval decreasing from 146 ms (81–258 ms) in the acute phase, to 133 ms (89–172 ms), p < 0.01 by first follow-up and then plateauing to 135 ms (95–173 ms), p = 0.29 by final follow-up, correcting appropriately with minor variations in heart rate across these time points. There were no ECGs demonstrating significant ST segment abnormalities or bundle branch block on final follow-up.
There were no documented arrhythmias detected during follow-up and no children presented with symptoms consistent with arrhythmias, with only four ambulatory monitors ordered between first and final follow-up clinics, all in asymptomatic children (requested for a finding of resting bradycardia in 2 children, previous non-sustained atrial tachycardia during admission in 1 child and symptoms of paroxysmal breath-holding events in an infant). Each of these monitors recorded 24 h of continuous ECG data and showed no arrhythmias.
Adverse Events
One patient presented with a large thrombus in the LV (see Fig. 3). In addition to immunomodulation the patient received intravenous unfractionated heparin. There was full resolution of the thrombus with no embolic complications. Two patients required extra-corporeal membrane oxygenation (ECMO) for cardiovascular support; one died from a large cerebrovascular accident (CVA) as a complication of the ECMO, the other was successfully decannulated and survived to 6 month follow-up with complete resolution of the cardiac sequelae. One patient developed typical chest pain with ECG changes and a large troponin rise. CMR demonstrated a small subendocardial infarct. This patient continues under long-term surveillance.
Discussion
The challenges of treating a new condition such as MIS-C have necessitated international collaboration and guidance by expert consensus [24]. Effective treatment algorithms initially borrowed heavily from similar inflammatory conditions such as Kawasaki Disease and acute Covid myocarditis. While some overlap in phenotype with other conditions such as Kawasaki disease shock syndrome (KDSS), and acute Covid myocarditis occurs, emerging studies suggest that MIS-C may be a separate disease with a distinctly higher incidence and specific distribution of cardiac complications [10]. Undoubtedly intensive first line treatment appears to successfully modulate the acute inflammatory biomarkers associated with defervescence [25] but to date there are only a small number of studies looking at the longer course of the disease, focusing on resolution of inflammation and with global measures of function used as an end-point rather than providing a full description of the course of the cardiac manifestations. In this follow-up study we specifically focused on the distribution of cardiac effects to show that children with MIS-C initially have evidence of significant cardiovascular involvement including coronary dilatation and myocardial impairment consistent with a pancarditis but with the majority complete resolution can be expected by 8 weeks.
We have previously shown that [11], cardiac dysfunction was worst at day 7 after the onset of the fever with resolving myocardial inflammation still evident two weeks later on MR imaging. Our current analysis of an extended cohort continues to support this observation. We detected cardiac dysfunction in 46.3% of patients using strain analysis with the worst 3D LV ejection fraction seen 6.4 days after the onset of the febrile illness. Studies utilizing multi-modality imaging suggest that the degree of cardiac involvement may be as high as 60% of patients [11]. In this larger follow-up study using echocardiographic data we found: endocardial involvement as evidenced by moderate (or worse) valvar regurgitation in 26.8%; myocardial dysfunction in 46.3% and ECG repolarisation changes in 15%; and pericardial effusions in 31.3% and a vasculitis with coronary dilatation in 26.8%. This degree of cardiac impairment is consistent with other reported case series [9, 26] indicating the high level of cardiac involvement contributing to the acuity of the illness. Some variation between the different functional indices (FS, EF, and strain) is observed and wholly expected as we see that MIS-C typically causes a regional wall motion abnormality preferentially affecting the basal LV septum and sparing the apical and mid segments of the LV. Therefore, regional wall motion analysis or 3D EF may be more sensitive markers of dysfunction than those that use single planar measurements which may miss the affected regional segments [11, 27, 28].
Electrocardiographic changes in children with MIS-C were best seen by comparing serial ECGs for each patient over time and were characterized by changes in T-wave amplitude and polarity in the precordial leads. We found flattened or inverted lateral T-waves showing recovery to normal patterns between the acute phase and first follow-up. Further medium-term follow-up confirmed normalization of T-wave flattening (measured objectively as a ratio to the R wave by using the T/R ratio) and recovery of those showing abnormal, lateral T-wave inversion. Although T/R ratio is used infrequently in clinical practice, for example to help predict outcomes in inferolateral early repolarization. Its use in our study was to better quantify relative T-wave amplitude and ‘flattening’ and we do not suggest a clinical application of the measurement in this patient group [29]. Final follow-up ECGs showed no major abnormalities and the small amount of ambulatory monitoring in later follow-up was unremarkable. Similar to echocardiographic findings, this suggests the most intense period of monitoring for electrocardiographic changes and arrhythmias should be the acute period and up to the first follow-up.
There has been some recent optimism with treating MIS-C. A recent large multi-center study comparing the outcomes of MIS-C with COVID estimated that 79% will have resolution of coronary aneurysms and 91% will have normalization of cardiac function within 30 days of onset [10]. A UK study has reported few organ-specific sequelae at 6 months [30]. Our study data builds on this evidence looking specifically at the changes in cardiac function, ECG changes and coronary involvement over a 6-month period. At the outset of this new disease experience, the medium-term outlook was unknown; therefore, all affected young people in our institution received structured follow-up for at least 6 months [9]. Reassuringly, almost all showed significant improvement, with normalization of cardiac function in all and regression of coronary dilatation in 98.5% within 6–8 weeks of the acute illness. Importantly no further improvement was seen at the 6 months assessment indicating an early return to baseline with no evidence of any relapses. Our group and others have used cardiac magnetic resonance (CMR) imaging and have found myocardial oedema as the underlying process [11]. As inflammation subsides, we would expect the acute myocardial oedema to subside and with this we are able to observe a concomitant improvement in both diastolic and systolic indices of cardiac function.
Furthermore we found that with immunomodulation and cessation of inflammation there was a reassuring improvement in the size of the coronary arteries in all children with small aneurysms or less by 8 weeks. A knowledge of the evolution of coronary dilatation in this condition is important as an early suspicion allows appropriate escalation of treatment to limit the dilatation. Interestingly the degree of coronary involvement may be higher than reported as we use an absolute Z score value of > 2 to delineate dilatation but some may not cross this threshold yet show a relative dilatation as evidenced by resolution in the size of the coronary arteries on serial imaging as the inflammation settles. This has been described for patients with KD [31]. This data compares well to descriptions of coronary dilatation occurring in febrile children. Previous data have shown coronary arteries may increase in size by + 0.5 standard deviations during the febrile portion of an illness before normalizing as the inflammatory cascade subsides [32].
One child had persistence of large aneurysms and minimal change in their small aneurysms over the 6 month course. The dilatation persisted in all affected vessels. This child was one of the first to present to our institution and their disease process may represent the more serious end of the spectrum of the natural course of this condition. The patient remains on warfarin anticoagulation to reduce the risk of thrombosis and continues to receive long-term follow-up. Two other major unavoidable adverse events were noted in this series of children. The first was a patient who was found to have a large LV thrombus detected on admission; the second was a sub-endocardial infarct in the infero-lateral basal septum found after symptoms of typical chest pain and ECG changes. These likely represent the pro-thrombotic tendency of the previous acute covid infection. We have since modified our treatment pathway to provide acute anti-coagulation to all patients hospitalized with MIS-C.
Conclusion
Pancarditis is a common finding in those with MIS-C, our data shows that after defervescence of inflammation the outcomes appear to offer promising results with resolution of cardiac findings in almost all patients with small aneurysms or less. In the current era of limited health resources, a restructuring of follow-up arrangements should be considered. In those with fully resolved uncomplicated MIS-C, follow-up arrangements could be rationalized to a single review at 6–8 weeks after the illness resolution followed by discharge if all findings are as expected and should lead to optimism with regard to prognostic counseling of these often highly traumatized young patients.
Limitations
This study was performed retrospectively. As a consequence of this being a new condition there was a wide variation in the immuno-modulation strategy and hence also the cardiac outcomes. However, recent evidence suggests there are a number of different effective immuno-modulation strategies and the focus should be attenuating the disease process in the first instance before severe inflammatory effects occur. We have focused on describing only the cardiac effects in detail. The next step would be to try and link these with other clinical and biochemical markers to attempt to risk stratify patients into appropriate treatments. We look forward to the results of the RECOVERY trial which continues to help guide us further on the most effective treatments [33].
A variety of treatment strategies have been used within our institution to good effect to immuno-modulate the inflammatory process. We have included these details for reference. The most appropriate treatment strategy continues to be assessed and we look forward to seeing the outcome of these studies. MIS-C is a worldwide phenomenon and in resource limited environments particularly where vaccination rates are still low and COVID and MIS-C are more prevalent, it may actually be more important that the condition is recognized early with early use of immuno-modulation as the outlook should be avoidance of severe dysfunction or large aneurysms which may represent the natural course of the disease and carry a high degree of morbidity and mortality. The results of our analysis of diastolic dysfunction were varied. The median E/e′ value was 7.4 despite a considerable number of patients having elevated values > 8 which would constitute diastolic dysfunction [34]. Furthermore there was no significant change to these values on the follow-up imaging. As the results were not meaningful it is not possible to draw conclusions form this dataset.
References
Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P (2020) Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395(10237):1607–1608
Viner RM, Ward JL, Hudson LD, Ashe M, Patel SV, Hargreaves D et al (2020) Systematic review of reviews of symptoms and signs of COVID-19 in children and adolescents. Arch Dis Child 106(8):802–807
Bassareo pp. (2021) Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the United Kingdom and Ireland: what is new? Lancet Reg Health Eur 3:100090
Prevention CfDCa (2020) Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). https://emergency.cdc.gov/han/2020/han00432.asp
Health RcoPaC (2020) Guidance—Paediatric multisystem inflammatory syndrome temporally associated with COVID-19
Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P et al (2020) Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 324(3):259–269
Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M et al (2020) An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395(10239):1771–1778
Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J et al (2020) Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med 26(11):1701–1707
Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A (2021) Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 180(2):307–322
Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H et al (2021) Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 325(11):1074–1087
Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E et al (2021) Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging 22(8):896–903
Regan W, O’Byrne L, Stewart K, Miller O, Pushparajah K, Theocharis P et al (2021) Electrocardiographic changes in children with multisystem inflammation associated with COVID-19: associated with coronavirus disease 2019. J Pediatr 234:27–32
Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S et al (2021) Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation 143(1):21–32
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK et al (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the American society of echocardiography pediatric and congenital heart disease council. J Am Soc Echocardiogr 23(5):465–495
Pettersen MD, Du W, Skeens ME, Humes RA (2008) Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr 21(8):922–934
Bernard A, Addetia K, Dulgheru R, Caballero L, Sugimoto T, Akhaladze N et al (2017) 3D echocardiographic reference ranges for normal left ventricular volumes and strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 18(4):475–483
Eidem BW, McMahon CJ, Cohen RR, Wu J, Finkelshteyn I, Kovalchin JP et al (2004) Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr 17(3):212–221
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA et al (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133
Levy PT, Machefsky A, Sanchez AA, Patel MD, Rogal S, Fowler S et al (2016) Reference ranges of left ventricular strain measures by two-dimensional speckle-tracking echocardiography in children: a systematic review and meta-analysis. J Am Soc Echocardiogr 29(3):209–225
Simpson J, Lopez L, Acar P, Friedberg M, Khoo N, Ko H et al (2016) Three-dimensional echocardiography in congenital heart disease: an expert consensus document from the European Association of Cardiovascular Imaging and the American society of echocardiography. Eur Heart J Cardiovasc Imaging 17(10):1071–1097
Perez-Casares A, Cesar S, Brunet-Garcia L, Sanchez-de-Toledo J (2017) Echocardiographic evaluation of pericardial effusion and cardiac tamponade. Front Pediatr 5:79
Dallaire F, Dahdah N (2011) New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr 24(1):60–74
Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH (2013) Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 26(2):185–191
Harwood R, Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan AV et al (2021) A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health 5(2):133–141
McArdle AJ, Vito O, Patel H, Seaby EG, Shah P, Wilson C et al (2021) Treatment of multisystem inflammatory syndrome in children. N Engl J Med 385(1):11–22
Ramcharan T, Nolan O, Lai CY, Prabhu N, Krishnamurthy R, Richter AG et al (2020) Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol 41(7):1391–1401
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al (2016) 016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37(27):2129–2200
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F et al (2016) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European society of cardiology (ESC). Eur Heart J 37(3):267–315
Roten L, Derval N, Maury P, Mahida S, Pascale P, Leenhardt A et al (2016) Benign vs. malignant inferolateral early repolarization: focus on the T wave. Heart Rhythm 13(4):894–902
Penner J, Abdel-Mannan O, Grant K, Maillard S, Kucera F, Hassell J et al (2021) 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health 5(7):473–482
McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M et al (2017) Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 135(17):e927–e999
Muniz JC, Dummer K, Gauvreau K, Colan SD, Fulton DR, Newburger JW (2013) Coronary artery dimensions in febrile children without Kawasaki disease. Circ Cardiovasc Imaging 6(2):239–244
Group RC (2021) Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397(10285):1637–1645
Choi SH, Eun LY, Kim NK, Jung JW, Choi JY (2016) Myocardial tissue doppler velocity in child growth. J Cardiovasc Ultrasound 24(1):40–47
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are employees of Guy’s and St Thomas’ NHS Foundation Trust and/or King’s College London. There are no competing interests or disclosures. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical Approval
Data were collected in accordance with the 1964 Helsinki declaration. Institutional ethics approval was obtained permitting retrospective review and use of echocardiographic data for clinical research and publication (09/H0802/116) without the need for informed written consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wong, J., Theocharis, P., Regan, W. et al. Medium-Term Cardiac Outcomes in Young People with Multi-system Inflammatory Syndrome: The Era of COVID-19. Pediatr Cardiol 43, 1728–1736 (2022). https://doi.org/10.1007/s00246-022-02907-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-02907-y