Abstract
We previously noted, in a small group of post-Fontan patients, a possible association between hepatic fibrosis scores and the status of pulmonary blood flow at birth. To further explore this observation, we examined data from all Fontan patients seen in our center from July 2010 to March 2015. We identified 200 patients for analysis. Of the 200 patients, 56 underwent transvenous-hepatic biopsy. Of the 200 patients, 13 (6.5 %) had protein-losing enteropathy. We divided both the 56 biopsy patients and the entire cohort of 200 patients into 4 groups: (1) unobstructed pulmonary blood flow at birth with functional left ventricles, (2) unobstructed pulmonary blood flow at birth with functional right ventricles, (3) obstructed pulmonary blood flow at birth with functional left ventricles, and (4) obstructed pulmonary blood flow at birth with functional right ventricles. Analysis of the 56 liver-biopsy patient groups showed median hepatic total-fibrosis scores for the 4 groups of 2 (0–6), 2 (0–8), 3 (2–6), and 4 (1–8), respectively, with statistical significance between groups 4 and 1 (p = 0.031). For the entire cohort of 200 patients, we analyzed the incidence of protein-losing enteropathy for each of the four groups and found protein-losing enteropathy percent occurrences of 0, 2.9, 8.8, and 16.1, respectively, with statistical significance between groups 4 and 2 (p = 0.031) and between groups 4 and 1 (p = 0.025). A history of obstructed pulmonary blood flow at birth, coupled with a functional right ventricle, may predict a poorer long-term Fontan outcome.
Similar content being viewed by others
Introduction
Hepatic fibrosis and protein-losing enteropathy (PLE) may develop after Fontan surgery [14]. Previous reports describe portal and sinusoidal hepatic fibrosis even in asymptomatic Fontan patients [2, 11]. Recently, we reported an association between hepatic total-fibrosis scores and postoperative Fontan patients with and without a history of obstructed pulmonary blood flow at birth [3]. This study analyzes data from all Fontan patients seen at our center over the last 5 years and reports differences between those born with unobstructed versus obstructed pulmonary blood flow, coupled with either a functional univentricle of left- or right-ventricular type.
Methods
We obtained data for this observational, retrospective study by inquiring our research database (Epi-Info™) and electronic health records (EHR) for patients with Fontan procedures seen at our center between July 2010 and March 2015. This study received approval from the local institutional review board. The Children’s Heart Center is the sole provider of congenital heart care in Nevada, and our database and EHR contain information on all patients with Fontan procedures seen in the state. For the searchable parts of our EHR, we used Perspective Software by Lexmark International, Inc. Lexington, KY, USA, to identify patient records containing the following search terms: Fontan, Glenn, protein-losing enteropathy, PLE, alpha-1-antitrypsin, hepatic or liver fibrosis, and hepatic or liver biopsy. Following inquiry of our research database and EHR, we reviewed patient records and collated data for analysis.
During routine Fontan follow-up, we obtained electrocardiograms, echocardiograms, complete blood counts with platelet quantification, serum liver function tests, renal function tests, and often, but not routinely, protein-C activity. In selected patients, we obtained FibroSure™ testing and stool for alpha-1-antitrypsin. FibroSure™ is a patented biomarker evaluation that uses six serum tests to render a score to predict liver fibrosis. We neither routinely obtained gamma-glutamyl transferase serum values nor pro-time values unless patients were on warfarin.
We defined obstructed pulmonary flow at birth as those requiring procedures to augment pulmonary blood flow and unobstructed flow as those requiring procedures that included restriction of pulmonary blood flow. We defined PLE as a serum albumin <3.5 g/dL, a fecal alpha-1-antitrypsin >55 mg/dL, or both.
Congenital cardiologists performed all cardiac catheterizations under general anesthesia, and prior to the completion of cardiac catheterization, interventional radiologists routinely performed transvenous-hepatic biopsies using the transjugular route and a 20-gauge biopsy system in patients ≥7 of age. All liver-biopsy specimens were stained with hematoxylin and eosin, trichrome, and reticulin. Two pathologists, with specialty training in gastrointestinal and liver pathology, reviewed each specimen independently. The pathologists were blinded to patient characteristics. Semiquantitative analysis was performed for portal fibrosis using the modified Scheuer staging system (0–4) and a previously employed staging system for sinusoidal fibrosis (0–4) [16]. If the two pathologists scored a specimen feature differently, then they conferred to arrive at a final score. For each patient, we added the portal-fibrosis score to the sinusoidal fibrosis score for a total-fibrosis score (0–8) [2].
For data analysis, we used SPSS version 13.0 (SPSS Inc., Chicago, Illinois, USA). We used both parametric and nonparametric tests for the statistical analysis. We set a p value of <0.05 as significant.
Results
For the period July 2010 through March 2015, we identified 200 post-Fontan surgical patients that underwent evaluation at our center. Average age was 14 years (2–44 years), 65 % were males, and average duration of Fontan was 10 years (0–35 years). Of the 200 patients, 23 (11.5 %) had heterotaxic situs: 14 with right atrial isomerism–asplenia, 6 with left atrial isomerism–polysplenia, and 3 with inversus. Of the 200 patients, laboratory findings were consistent with PLE in 13 (6.5 %). Of the 200 patients, 183 (91.5 %) are active. Of the 17 patients not currently active, 13 moved to another state, and 4 died: 2 PLE related, 1 from influenza A, and 1 late postoperative.
Table 1 lists all patients’ initial Fontan types and the outcome breakdown for fenestrations in both extracardiac and lateral tunnels. For those that underwent cardiac catheterization, Table 2 lists the type of interventions performed and numbers undergoing transvenous-liver biopsies. Transvenous-hepatic-biopsy procedures added <10 min to cardiac catheterizations. There were no complications in any of the 56 patients. The average liver-biopsy specimen was 1.5 cm in length (1.1–2.1 cm). All specimens were deemed interpretable, and none had a significant inflammation.
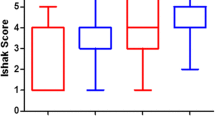
Table 3 divides the findings from 56 patients that underwent transvenous-hepatic biopsy into four groups: (1) unobstructed pulmonary blood flow at birth with functional left ventricles, (2) unobstructed pulmonary blood flow at birth with functional right ventricles, (3) obstructed pulmonary blood flow at birth with functional left ventricles, and (4) obstructed pulmonary blood flow at birth with functional right ventricles. A Kruskal–Wallis H test showed that there was a statistically significant difference in hepatic total-fibrosis scores between the four groups (p = 0.031), and a pairwise multiple comparison t test (Dunnett) demonstrated the control group (group 4) comprised statistically significant higher hepatic total-fibrosis scores than group 1 (p = 0.033) but not statistically significant higher scores when compared to groups 2 and 3.
For the entire cohort of 56 liver-biopsy patients, there were no statistically significant correlations between hepatic total-fibrosis scores and total serum bilirubin, total protein, albumin, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, creatinine, hemoglobin, hematocrit, or platelet count. Further, we found no significant correlations between hepatic total-fibrosis scores and inferior vena caval pressure, transpulmonary-pressure gradients, Fick-calculated pulmonary vascular resistance, functional-univentricle end-diastolic pressure, Fick-calculated systemic blood flow, or duration of Fontan. The only statistically significant correlation we found between the hepatic total-fibrosis scores, for the liver-biopsy cohort as a whole, was an inverse correlation between total scores and oxygen saturation.
Table 4 similarly divides all 200 patients into four groups in the manner we divided the biopsy patients in Table 3. An ANOVA with post hoc test showed that group 4 had a statistically significant higher incidence of PLE when compared to groups 1 (p = 0.03) and 2 (p = 0.03), but not with group 3.
Table 5 compares the PLE patients, as a single cohort, with the entire cohort without PLE. Those with PLE had significant differences, including higher liver total-fibrosis scores median values, older ages, longer Fontan duration times, more pacemakers, more with decreased univentricular function, more with atrioventricular valve regurgitation, and higher inferior vena caval pressures. There were no differences in percentages of males, oxygen saturation, nor univentricular end-diastolic pressure or pulmonary vascular resistance at cardiac catheterization.
Table 6 tabulates the laboratory values for the 13 patients with PLE and 135 without PLE for which we had complete data. Of the 13 PLE patients, 11 were diagnosed by hypoalbuminemia, 1 had hypoalbuminemia and increased fecal alpha-1-antitrypsin, and 1 had only increased fecal alpha-1-antitrypsin but a normal albumin level. The one patient with an elevated fecal alpha-1-antitrypsin had a serum albumin of 4.4 g/dL, which is reflected in the range of albumin levels for the PLE patients in Table 6. We did not routinely test for fecal alpha-1-antitrypsin in all Fontan patients; nonetheless, of the 19 values obtained in 19 different patients, only 2 were elevated. Both median albumin and total proteins were significantly lower in patients with PLE. There were no significant differences in values for hemoglobin, hematocrit, platelet count, aminotransferase, alanine aminotransferase, and total bilirubin levels between those with and without PLE. No patient seen during the study period presented with plastic bronchitis.
Additional laboratory values included protein-C activity and FibroSure™ testing.
Of the 58 values obtained for protein-C activity, none was abnormal; further, the protein-C activity values neither correlated with other parameters nor were they useful in screening for protein-losing enteropathy. We performed FibroSure™ testing in 8 age-appropriate patients (≥14 years) for analysis, in conjunction with transvenous-liver biopsies. We found only 1 FibroSure™ value consistent with a hepatic portal-fibrosis score obtained by biopsy.
Discussion
We previously speculated that the higher hepatic total-fibrosis scores in those with a history of obstructed pulmonary flow at birth might be related to differences in pulmonary vascular resistance secondary to pulmonary vascular development [3]. Differences in pulmonary vascular development may be related to the findings of reduced number of intra-acinar arteries in neonates born with pulmonary atresia versus an increased number of intra-acinar arteries in newborns born with aortic atresia [6, 7, 13, 17]. Main left- and right-pulmonary artery and Fontan-pathway distortions could also play a role. In our series, however, we noted pulmonary artery and Fontan stents placed and redilated in an equal number of patients with and without a history of pulmonary blood flow obstruction at birth.
In the Fontan circulation, Fick-calculated pulmonary vascular resistance is likely an imprecise measurement [10]. Additionally, the liver is exposed not simply to isolated pulmonary vascular resistance but to Fontan-circuit vascular resistance from the inferior vena cava to the distal pulmonary arteries. Subtle differences in the entire Fontan-circuit vascular resistance, at similar pulmonary artery, Fontan, and inferior vena caval pressures, may contribute to the differences in hepatic-biopsy findings found in those with a history of pulmonary blood flow obstruction versus unobstructed pulmonary blood flow at birth. More precise methods of measuring the entire Fontan-circuit vascular resistance, other than via pressure, flow, and the Fick equation with assumed oxygen consumption, will be necessary to explore the hypothesis that subtle differences in vascular resistance may play a role in the development of hepatic fibrosis.
Mitchell et al. [12] noted pulmonary vascular resistance differences in the same Fontan patient pre- and post-heart transplant. They found that pulmonary vascular resistance in the pre-heart transplant Fontan patient increased significantly after transplantation, as the new circulatory dynamics increased transpulmonary pressures, which unmasked previously elevated pulmonary vascular resistance in Fontan patients but undetected because of decreased cardiac output and impaired pulmonary blood flow. Also of note in Mitchell et al’s [12] report, 8/11 (73 %) of their tabulated late-Fontan failure group had functional-univentricular hearts with either neonatal pulmonary atresia or pulmonary stenosis.
Some reports suggest heterotaxy is a risk factor for PLE [9]. Pulmonary outlet obstruction is common in heterotaxy patients. Our review of heterotaxy in Southern Nevada found 75 % of the functional-univentricular hearts had pulmonary stenosis or pulmonary atresia [4]. Previous reports also suggest that a functional-univentricle of right-ventricular type is a risk factor for PLE [15]. Thus, despite ventricular dysfunction, atrioventricular valve regurgitation, and others, obstructed pulmonary flow at birth may be a compounding additional risk factor for PLE in heterotaxy patients and those with functional univentricles of right-ventricular type.
The symptoms, signs, and laboratory abnormalities of PLE can be ameliorated by pharmacological, cardiac-catheter interventional techniques, and surgery including heart transplantation [1, 8]. Nonetheless, the liver abnormalities induced by Fontan physiology may persist. In contrast to inflammatory causes, mild and even moderate hepatic fibrosis, secondary to a Fontan procedure, is often associated with essentially normal liver function [5]. Nevertheless, our data suggest that, once PLE occurs, hepatic fibrosis will often be advanced.
Similar to all retrospective, observational reports, this study has limitations. Fontan investigations are complicated by the heterogeneous anatomy and physiology of functional-univentricular hearts. Liver-biopsy grading is semiquantitative, and patchy pathology can lead to sampling errors. However, since March 2012, transvenous-hepatic biopsy has been part of our routine cardiac catheterizations on all Fontan patients ≥7 years of age; thus, our biopsy population is not skewed toward symptomatic patients.
In conclusion, our data suggest that, in Fontan-palliated patients, a history of obstructed pulmonary blood flow at birth, coupled with a univentricle of right-ventricular type, may be combination that portends a poorer long-term outcome than others. Analysis of data from other Fontan populations will be needed to determine whether our observations are reproducible.
References
Bejiqi R, Retkoceri R, Zeka N, Bejiqi H, Vuqiterna A, Maloku A (2014) Treatment of children with protein-losing enteropathy after Fontan and other complex congenital heart disease procedures in condition with limited human and technical resources. Mater Sociomed 26:39–42
Evans WN, Winn BJ, Yumiaco NS, Galindo A, Rothman A, Acherman RJ, Restrepo H (2014) Transvenous hepatic biopsy in stable Fontan patients undergoing cardiac catheterization. Pediatr Cardiol 35:1273–1278
Evans WN, Acherman RJ, Winn BJ, Yumiaco NS, Galindo A, Rothman A, Restrepo H (2015) Fontan hepatic fibrosis and pulmonary vascular development. Pediatr Cardiol 36:657–661
Evans WN, Acherman RJ, Restrepo H (2015) Heterotaxy in Southern Nevada: prenatal detection and epidemiology. Pediatr Cardiol 36(5):930–934
Guha IN, Bokhandi S, Ahmad Z, Sheron N, Cope R, Marshall C, Veldtman G (2013) Structural and functional uncoupling of liver performance in the Fontan circulation. Int J Cardiol 164:77–81
Haworth SG, Reid L (1977) Quantitative structural study of pulmonary circulation in the newborn with pulmonary atresia. Thorax 32:129–133
Haworth SG, Reid L (1977) Quantitative structural study of pulmonary circulation in the newborn with aortic atresia, stenosis, or coarctation. Thorax 32:121–128
John AS, Johnson JA, Khan M, Driscoll DJ, Warnes CA, Cetta F (2014) Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J Am Coll Cardiol 64:54–62
Johnson JA, Cetta F, Graham RP, Smyrk TC, Driscoll DJ, Phillips SD, John AS (2013) Identifying predictors of hepatic disease in patients after the Fontan operation: a postmortem analysis. J Thorac Cardiovasc Surg 146:140–145
Kaza AK, Kaza E, Bullock E, Reyna S, Yetman A, Everitt MD (2015) Pulmonary vascular remodelling after heart transplantation in patients with cavopulmonary connection. Eur J Cardiothorac Surg 47:505–510
Kendall TJ, Stedman B, Hacking N, Haw M, Vettukattill JJ, Salmon AP, Cope R, Sheron N, Millward-Sadler H, Veldtman GR, Iredale JP (2008) Hepatic fibrosis and cirrhosis in the Fontan circulation: a detailed morphological study. J Clin Pathol 61:504–508
Mitchell MB, Campbell DN, Ivy D, Boucek MM, Sondheimer HM, Pietra B, Das BB, Coll JR (2004) Evidence of pulmonary vascular disease after heart transplantation for Fontan circulation failure. J Thorac Cardiovasc Surg 128:693–702
Ruchonnet-Metrailler I, Bessieres B, Bonnet D, Vibhushan S, Delacourt C (2014) Pulmonary hypoplasia associated with congenital heart diseases: a fetal study. PLoS One 9:e93557
Rychik J, Goldberg DJ (2014) Late consequences of the Fontan operation. Circulation 130:1525–1528
Schumacher KR, Stringer KA, Donohue JE, Yu S, Shaver A, Caruthers RL, Zikmund-Fisher BJ, Fifer C, Goldberg C, Russell MW (2015) Fontan-associated protein-losing enteropathy and plastic bronchitis. J Pediatr 166(4):970–977
Schwartz MC, Sullivan LM, Glatz AC et al (2013) Portal and sinusoidal fibrosis are common on liver biopsy after Fontan surgery. Pediatr Cardiol 34:135–142
Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, Haworth SG (2009) Development and pathology of pulmonary hypertension. J Am Coll Cardiol 54:S3–S9
Acknowledgments
We wish to thank the interventional radiologists of Radiology Specialists at Sunrise Children’s Hospital and Medical Center, who performed the transvenous-hepatic biopsies, including Drs. Demetrice Davis, Steven Davis, Kelly Gardner, Sunil Gujrathi, Aaron Peterson, and Matthew Rainey.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evans, W.N., Acherman, R.J., Reardon, L.C. et al. Fontan Outcomes and Pulmonary Blood Flow at Birth. Pediatr Cardiol 37, 30–36 (2016). https://doi.org/10.1007/s00246-015-1234-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-015-1234-1